Hyperpolarization induces a long-term increase in the spontaneous firing rate of cerebellar Golgi cells - PubMed (original) (raw)
Hyperpolarization induces a long-term increase in the spontaneous firing rate of cerebellar Golgi cells
Court A Hull et al. J Neurosci. 2013.
Abstract
Golgi cells (GoCs) are inhibitory interneurons that influence the cerebellar cortical response to sensory input by regulating the excitability of the granule cell layer. While GoC inhibition is essential for normal motor coordination, little is known about the circuit dynamics that govern the activity of these cells. In particular, although GoC spontaneous spiking influences the extent of inhibition and gain throughout the granule cell layer, it is not known whether this spontaneous activity can be modulated in a long-term manner. Here we describe a form of long-term plasticity that regulates the spontaneous firing rate of GoCs in the rat cerebellar cortex. We find that membrane hyperpolarization, either by mGluR2 activation of potassium channels, or by somatic current injection, induces a long-lasting increase in GoC spontaneous firing. This spike rate plasticity appears to result from a strong reduction in the spike after hyperpolarization. Pharmacological manipulations suggest the involvement of calcium-calmodulin-dependent kinase II and calcium-activated potassium channels in mediating these firing rate increases. As a consequence of this plasticity, GoC spontaneous spiking is selectively enhanced, but the gain of evoked spiking is unaffected. Hence, this plasticity is well suited for selectively regulating the tonic output of GoCs rather than their sensory-evoked responses.
Figures
Figure 1.
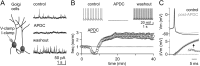
Transient activation of GoC mGluR2 receptors increases spontaneous inhibition by producing a long-term increase in tonic spiking. A, Left, recording configuration for all experiments. Right, Voltage-clamp recording of sIPSCs at 0 mV (top). Example recording shows that the mGluR2 agonist APDC reduces the sIPSC rate (middle), and upon washout the sIPSC rate is elevated (bottom). B, Top, Current-clamp recording reveals that APDC eliminates GoC spontaneous spiking, and spike rate is elevated upon washout. Bottom, Summary time course showing the effect of transient mGluR2 activation on the GoC spike rate (n = 8). C, Average AP waveform in control (top, black) and 15 min after APDC (top, gray) reveals a reduction in the AHP (n = 8). Bottom, Subtracted AP waveforms illustrating the mean difference (±SEM) quantified 20 ms after the AP peak (ΔVm20ms).
Figure 2.
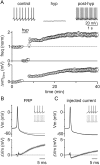
Transient hyperpolarization induces a long-term increase in GoC spiking and a corresponding decrease in spike AHPs. A, Top, Example experiment showing that a 3 min negative current injection of −50 pA hyperpolarizes the GoC membrane to ∼−65 mV and suppresses spiking. An increase in GoC spiking occurred after hyperpolarization. Bottom, Summary of all experiments (n = 20) where GoCs were hyperpolarized with current injection shows a long-term increase in spontaneous spiking and a decrease in AHP amplitudes. B, Mean AP waveform before (top, black) and after (top, gray) hyperpolarization, and difference waveforms (bottom) reveal a reduction in AHPs (n = 20). Inset, 1 s of spiking from an example cell before and after induction of FRP with a 3 min current injection of −75 pA. C, Current injection was used to adjust GoC spike to the average mean rates observed before and after hyperpolarization in A. Averaged APs at low frequency (4.4 ± 0.1 Hz, top black), high frequency (7.7 ± 0.1 Hz, top gray), and the mean difference trace (bottom, black) reveal little change in the waveforms at low and high frequency. Inset, 1 s of spiking from an example cell before and after a 7 pA current injection.
Figure 3.
FRP involves CaMKII and BK-type potassium channels. Current-clamp experiments were performed in which the spontaneous firing of GoCs and the GoC spike waveform was monitored. A, C, E, The wash-in effect of the CaMKII inhibitor KN-62 (A, n = 7), the SK-type potassium channel antagonist apamin (C, n = 7), and the BK-type potassium channel inhibitor paxilline (E, n = 10) was assessed. B, D, F, The effect of a 3 min somatic hyperpolarization was also assessed in slices incubated in the presence of KN-62 (B, n = 6), apamin (D, n = 10), and paxilline (F, n = 17).
Figure 4.
GoC FRP involves a subset of BK-type potassium channels. A, Left, Paxilline was applied after the induction of FRP (n = 7). Right, FRP reduced the spike AHP (blue), whereas paxilline selectively broadened the AP (red). B, Comparison of the difference waveforms for paxilline applied without FRP induction (Fig. 3, black) and after FRP induction (red) reveals that paxilline has a much larger effect on the AHP (later time points) when applied without inducing FRP. The paxilline-sensitive component of FRP is small and late in the AHP (double arrow). C, Iberiotoxin wash-in elevates the spike rate of GoCs and selectively decreases the spike AHP. D, Iberiotoxin incubation does not occlude FRP, and the spike AHP is still significantly reduced after induction of plasticity.
Figure 5.
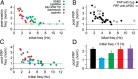
The magnitude of GoC FRP depends on initial firing rate. A, In all drug wash-ins, the increase in firing rate was greatest for cells with low initial firing rates. Little or no increase in firing rate was observed for initial firing rates >5 Hz. B, The magnitude of FRP was also dependent on initial firing rates, with lower firing rates producing the largest FRP. However, FRP was present at initial rates >5 Hz. C, For most drug conditions, FRP was also dependent on initial firing rate, and little or no FRP was evident for initial firing rates >5 Hz. D, Summary of FRP magnitude for cells with initial firing rates <5 Hz. Whereas FRP was still occluded by KN62, the effect of paxilline was reduced in cells with lower initial firing rates.
Figure 6.
Time dependence of FRP induction. A, GoC spike rates were recorded after hyperpolarizations of 0 (n = 8), 5 (n = 6), 20 (n = 10), 60 (n = 15), and 180 (n = 22) seconds. Although 5 s hyperpolarizations did not increase spike rates, longer durations each produced a nearly twofold increase in spiking. Data points represent 20 s intervals, resulting in a non-0 spiking value for 5 s of hyperpolarization B, Summary of GoC spike rates as a function of hyperpolarization time.
Figure 7.
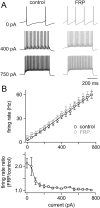
FRP alters the offset but not the gain of GoC input/output transformations. A, Example experiment showing the responses to injected current for a GoC before (black) and 15 min after (gray) inducing FRP with a 3 min hyperpolarization. Although FRP doubles the spontaneous spike rate, it has little influence on responses to injected current. B, Top, Summary of input/output relationships for injected current before and after inducing FRP with hyperpolarization. Lines represent linear fits to the averaged data (n = 10). The mean slope before hyperpolarization was 0.078 ± 0.006 Hz/pA, and 15 min after hyperpolarization was 0.077 ± 0.007 Hz/pA, indicating that FRP does not change the gain of transmission. Bottom, Ratio of the firing rate evoked by a current injection (after inducing FRP)/(before inducing FRP) reveals that the largest increase in spiking after induction of FRP occurs with no injected current, and this difference falls off rapidly with increasing current steps.
Similar articles
- Dynamics of fast and slow inhibition from cerebellar golgi cells allow flexible control of synaptic integration.
Crowley JJ, Fioravante D, Regehr WG. Crowley JJ, et al. Neuron. 2009 Sep 24;63(6):843-53. doi: 10.1016/j.neuron.2009.09.004. Neuron. 2009. PMID: 19778512 Free PMC article. - Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex.
Duguid I, Branco T, London M, Chadderton P, Häusser M. Duguid I, et al. J Neurosci. 2012 Aug 8;32(32):11132-43. doi: 10.1523/JNEUROSCI.0460-12.2012. J Neurosci. 2012. PMID: 22875944 Free PMC article. - Active Dendrites and Differential Distribution of Calcium Channels Enable Functional Compartmentalization of Golgi Cells.
Rudolph S, Hull C, Regehr WG. Rudolph S, et al. J Neurosci. 2015 Nov 25;35(47):15492-504. doi: 10.1523/JNEUROSCI.3132-15.2015. J Neurosci. 2015. PMID: 26609148 Free PMC article. - Pre- and postsynaptic inhibition mediated by GABA(B) receptors in cerebellar inhibitory interneurons.
Mann-Metzer P, Yarom Y. Mann-Metzer P, et al. J Neurophysiol. 2002 Jan;87(1):183-90. doi: 10.1152/jn.00344.2001. J Neurophysiol. 2002. PMID: 11784741 - Excitation of rat cerebellar Golgi cells by ethanol: further characterization of the mechanism.
Botta P, Simões de Souza FM, Sangrey T, De Schutter E, Valenzuela CF. Botta P, et al. Alcohol Clin Exp Res. 2012 Apr;36(4):616-24. doi: 10.1111/j.1530-0277.2011.01658.x. Epub 2011 Oct 17. Alcohol Clin Exp Res. 2012. PMID: 22004123 Free PMC article.
Cited by
- Physiology of Cerebellar Reserve: Redundancy and Plasticity of a Modular Machine.
Mitoma H, Kakei S, Yamaguchi K, Manto M. Mitoma H, et al. Int J Mol Sci. 2021 Apr 30;22(9):4777. doi: 10.3390/ijms22094777. Int J Mol Sci. 2021. PMID: 33946358 Free PMC article. Review. - Integrated plasticity at inhibitory and excitatory synapses in the cerebellar circuit.
Mapelli L, Pagani M, Garrido JA, D'Angelo E. Mapelli L, et al. Front Cell Neurosci. 2015 May 5;9:169. doi: 10.3389/fncel.2015.00169. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25999817 Free PMC article. Review. - Cerebellar Non-Invasive Brain Stimulation: A Frontier in Chronic Pain Therapy.
Sveva V, Cruciani A, Mancuso M, Santoro F, Latorre A, Monticone M, Rocchi L. Sveva V, et al. J Pers Med. 2024 Jun 23;14(7):675. doi: 10.3390/jpm14070675. J Pers Med. 2024. PMID: 39063929 Free PMC article. Review. - Acetylcholine Modulates Cerebellar Granule Cell Spiking by Regulating the Balance of Synaptic Excitation and Inhibition.
Fore TR, Taylor BN, Brunel N, Hull C. Fore TR, et al. J Neurosci. 2020 Apr 1;40(14):2882-2894. doi: 10.1523/JNEUROSCI.2148-19.2020. Epub 2020 Feb 28. J Neurosci. 2020. PMID: 32111698 Free PMC article. - Large conductance voltage-and calcium-activated K+ (BK) channel in health and disease.
Echeverría F, Gonzalez-Sanabria N, Alvarado-Sanchez R, Fernández M, Castillo K, Latorre R. Echeverría F, et al. Front Pharmacol. 2024 Mar 22;15:1373507. doi: 10.3389/fphar.2024.1373507. eCollection 2024. Front Pharmacol. 2024. PMID: 38584598 Free PMC article. Review.
References
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. - PubMed
- Chadderton P, Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F30 DC013716/DC/NIDCD NIH HHS/United States
- R01 NS032405/NS/NINDS NIH HHS/United States
- R37 NS032405/NS/NINDS NIH HHS/United States
- T32 MH020017/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials