A DR6/p75(NTR) complex is responsible for β-amyloid-induced cortical neuron death - PubMed (original) (raw)
A DR6/p75(NTR) complex is responsible for β-amyloid-induced cortical neuron death
Y Hu et al. Cell Death Dis. 2013.
Abstract
The p75 neurotrophin receptor (p75(NTR)) is a known mediator of β-amyloid (Aβ)-induced neurotoxicity implicated in Alzheimer's disease (AD). Here, we demonstrate that death receptor 6 (DR6) binds to p75(NTR) and is a component of the p75(NTR) signaling complex responsible for Aβ-induced cortical neuron death. Cortical neurons isolated from either DR6 or p75(NTR) null mice are resistant to Aβ-induced neurotoxicity. Blocking DR6 function in cortical neurons by anti-DR6 antibodies that block the binding of DR6 to p75(NTR) receptor complex or by a dominant negative DR6 construct lacking the cytoplasmic signaling death domain attenuates Aβ-induced caspase 3 activation and cell death. DR6 expression is upregulated in AD cortex and correlates with elevated neuronal death. Targeting the disruption of the DR6/p75(NTR) complex to prevent Aβ cytotoxicity represents a new approach for the treatment of neurodegenerative disorders such as AD.
Figures
Figure 1
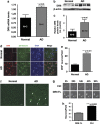
DR6 is expressed in cortical neurons and upregulated in AD. (a) Quantitative RT-PCR analysis of DR6 mRNA expression in AD. (b) Western blot analysis of DR6 expression from four AD and three age-matched normal brains. (c) Densitometry quantification of DR6 protein level from DR6 western blotting from 10 AD and 9 age-matched normal brains. (d) In-situ hybridization analysis of DR6 (red) and co-localization with neurons (_β_III-tubulin, green) in normal and AD cortex (yellow=merge, blue=DAPI). Arrows indicate cells with nuclear condensation characteristic of apoptosis. Arrowheads indicate area of image enlargement (top right corner). Scale bar=25 _μ_m. (e) Quantification of DR6+ neurons from d. (f) Immunocytochemical staining of DR6 from age-matched normal and AD brains; arrows indicate intensely stained DR6-positive cells. Scale bar=95 _μ_m. (g) Time-lapse images showing the effect of full-length DR6 (DR6 FL) overexpression in cultured neurons 0–92 h after transfection (bottom panel) versus vector control-transfected neurons (top panel). Scale bar=20 _μ_m. (h) Quantification of neuronal survival from g
Figure 2
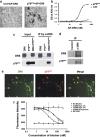
DR6 and p75NTR form a receptor complex. (a) AP-DR6 binding to HEK 293 cells expressing p75NTR and not to untransfected control cells. The non-homogenous staining pattern reflects incomplete transfection. Scale bar=25 _μ_m. (b) Quantitative assessment of a by ELISA. (c) DR6/p75NTR co-immunoprecipitation in HEK 293 cells overexpressing Myc-tagged DR6 and p75NTR. (d) DR6/p75NTR co-immunoprecipitation in fetal human spinal cord lysates. (e) Immunocytochemical staining to co-localized the DR6 and p75NTR expression in cultured neurons. Scale bar=25 _μ_m. (f) Competition ELISA showing the binding of europium-labeled human p75NTR to human DR6 ectodomain-coated plates in the presence and absence of blocker constructs
Figure 3
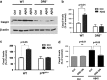
Cortical neurons isolated from DR6 and p75NTR null mice are resistant to A_β_42-induced cell death. (a) Western blot analysis of cleaved casp3 levels in DR6−/− cortical neurons after A_β_42 exposure. (b) Quantification of cleaved casp3 levels from a. (c) Quantification of cleaved casp3 levels by western blot analysis in p75NTR−/− cortical neurons after A_β_42 exposure. (d) Quantification of cleaved casp3 levels by western blot analysis in DR6-DN-infected neurons after A_β_42 exposure
Figure 4
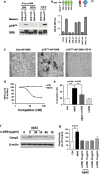
Anti-DR6 antibodies that block the interaction between DR6 and p75NTR inhibit A_β_42-induced neuronal cell death. (a) Anti-DR6 antibody 5D10 blocked DR6 and p75NTR complex formation from DR6/p75NTR co-transfected HEK 293 cells. Anti-DR6 antibody 2A9, which does not bind to DR6 at the p75NTR-binding site, did not block p75NTR from co-immunoprecipitating with DR6. (b) Top: Schematic domain structure of human DR6 protein depicting four extracellular CRD 1-4, a transmembrane domain (TM), and a cytoplasmic death domain (DD). Bottom: ELISA mapping 5D10-binding epitope within cysteine-rich domains of DR6 protein. (c) 5D10 blocks AP-DR6 binding to HEK 293 cells expressing p75NTR. Scale bar=25 _μ_m. (d) Quantitative assessment of 5D10 blocking AP-DR6 binding to p75NTR from c. (e) TUNEL staining quantification of percent apoptotic cells from anti-DR6 antibody-treated and control-treated cortical neurons following A_β_42 exposure. (f) Western blot analysis of cleaved casp3 levels in anti-DR6 antibody-treated and control-treated cortical neurons following A_β_42 exposure. (g) Quantification of cleaved casp3 levels from f
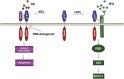
Figure 5
Working model for DR6/p75NTR receptor complex signaling in cortical neurons. In the presence of NTs, p75NTR binds NTs and Trk to promote cortical neuron survival via the AKT (also known as Protein Kinase B) pathway. In the absence of NTs, p75NTR binds A_β_ and DR6 to form a receptor complex, which activates the caspase 3 apoptotic signaling pathway via a cytoplasmic death domain oligomeric complex. In Alzheimer's disease, various factors might switch p75NTR signaling from pro-survival to pro-death, including the increased level of A_β_, upregulation of DR6 and p75NTR, and decreased expression of Trk
Similar articles
- The p75 neurotrophin receptor promotes amyloid-beta(1-42)-induced neuritic dystrophy in vitro and in vivo.
Knowles JK, Rajadas J, Nguyen TV, Yang T, LeMieux MC, Vander Griend L, Ishikawa C, Massa SM, Wyss-Coray T, Longo FM. Knowles JK, et al. J Neurosci. 2009 Aug 26;29(34):10627-37. doi: 10.1523/JNEUROSCI.0620-09.2009. J Neurosci. 2009. PMID: 19710315 Free PMC article. - Role of p75 neurotrophin receptor in the neurotoxicity by beta-amyloid peptides and synergistic effect of inflammatory cytokines.
Perini G, Della-Bianca V, Politi V, Della Valle G, Dal-Pra I, Rossi F, Armato U. Perini G, et al. J Exp Med. 2002 Apr 1;195(7):907-18. doi: 10.1084/jem.20011797. J Exp Med. 2002. PMID: 11927634 Free PMC article. - Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat.
Park JA, Lee JY, Sato TA, Koh JY. Park JA, et al. J Neurosci. 2000 Dec 15;20(24):9096-103. doi: 10.1523/JNEUROSCI.20-24-09096.2000. J Neurosci. 2000. PMID: 11124986 Free PMC article. - The killing of neurons by beta-amyloid peptides, prions, and pro-inflammatory cytokines.
Chiarini A, Dal Pra I, Whitfield JF, Armato U. Chiarini A, et al. Ital J Anat Embryol. 2006 Oct-Dec;111(4):221-46. Ital J Anat Embryol. 2006. PMID: 17385278 Review. - The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system.
Dechant G, Barde YA. Dechant G, et al. Nat Neurosci. 2002 Nov;5(11):1131-6. doi: 10.1038/nn1102-1131. Nat Neurosci. 2002. PMID: 12404007 Review.
Cited by
- Death receptor 6 (DR6) antagonist antibody is neuroprotective in the mouse SOD1G93A model of amyotrophic lateral sclerosis.
Huang G, Lee X, Bian Y, Shao Z, Sheng G, Pepinsky RB, Mi S. Huang G, et al. Cell Death Dis. 2013 Oct 10;4(10):e841. doi: 10.1038/cddis.2013.378. Cell Death Dis. 2013. PMID: 24113175 Free PMC article. - Multi-Arm PEG/Peptidomimetic Conjugate Inhibitors of DR6/APP Interaction Block Hematogenous Tumor Cell Extravasation.
Wang L, Shen Q, Liao H, Fu H, Wang Q, Yu J, Zhang W, Chen C, Dong Y, Yang X, Guo Q, Zhang J, Zhang J, Zhang W, Lin H, Duan Y. Wang L, et al. Adv Sci (Weinh). 2021 Jun;8(11):e2003558. doi: 10.1002/advs.202003558. Epub 2021 Mar 18. Adv Sci (Weinh). 2021. PMID: 34105277 Free PMC article. - Neuronal Cell Death.
Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Fricker M, et al. Physiol Rev. 2018 Apr 1;98(2):813-880. doi: 10.1152/physrev.00011.2017. Physiol Rev. 2018. PMID: 29488822 Free PMC article. Review. - Systematic elucidation of neuron-astrocyte interaction in models of amyotrophic lateral sclerosis using multi-modal integrated bioinformatics workflow.
Mishra V, Re DB, Le Verche V, Alvarez MJ, Vasciaveo A, Jacquier A, Doulias PT, Greco TM, Nizzardo M, Papadimitriou D, Nagata T, Rinchetti P, Perez-Torres EJ, Politi KA, Ikiz B, Clare K, Than ME, Corti S, Ischiropoulos H, Lotti F, Califano A, Przedborski S. Mishra V, et al. Nat Commun. 2020 Nov 4;11(1):5579. doi: 10.1038/s41467-020-19177-y. Nat Commun. 2020. PMID: 33149111 Free PMC article. - How Does p73 Cause Neuronal Defects?
Niklison-Chirou MV, Killick R, Knight RA, Nicotera P, Melino G, Agostini M. Niklison-Chirou MV, et al. Mol Neurobiol. 2016 Sep;53(7):4509-20. doi: 10.1007/s12035-015-9381-1. Epub 2015 Aug 13. Mol Neurobiol. 2016. PMID: 26266644 Review.
References
- Yan Z, Feng J. Alzheimer's disease: interactions between cholinergic functions and beta-amyloid. Curr Alzheimer Res. 2004;1:241–248. - PubMed
- Mahmood Z, Shukla Y. Death receptors: targets for cancer therapy. Exp Cell Res. 2010;316:887–899. - PubMed
- Mi S, Lee X, Hu Y, Ji B, Shao Z, Yang W, et al. Death receptor 6 negatively regulates oligodendrocyte survival, maturation and myelination. Nat Med. 2011;17:816–821. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials