Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial - PubMed (original) (raw)
Clinical Trial
. 2013 Sep;36(9):2508-15.
doi: 10.2337/dc12-2491. Epub 2013 Apr 5.
Affiliations
- PMID: 23564919
- PMCID: PMC3747923
- DOI: 10.2337/dc12-2491
Clinical Trial
Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial
Guntram Schernthaner et al. Diabetes Care. 2013 Sep.
Erratum in
- Diabetes Care. 2013 Dec;36(12):4172
Abstract
Objective: To evaluate the efficacy and safety of canagliflozin, a sodium glucose cotransporter 2 inhibitor, compared with sitagliptin in subjects with type 2 diabetes inadequately controlled with metformin plus sulfonylurea.
Research design and methods: In this 52-week, randomized, double-blind, active-controlled, phase 3 study, subjects using stable metformin plus sulfonylurea (N = 755) received canagliflozin 300 mg or sitagliptin 100 mg daily. Primary end point was change from baseline in A1C at 52 weeks. Secondary end points included change in fasting plasma glucose (FPG) and systolic blood pressure (BP), and percent change in body weight, triglycerides, and HDL cholesterol. Safety was assessed based on adverse event (AE) reports.
Results: At 52 weeks, canagliflozin 300 mg demonstrated noninferiority and, in a subsequent assessment, showed superiority to sitagliptin 100 mg in reducing A1C (-1.03% [-11.3 mmol/mol] and -0.66% [-7.2 mmol/mol], respectively; least squares mean difference between groups, -0.37% [95% CI, -0.50 to -0.25] or -4.0 mmol/mol [-5.5 to -2.7]). Greater reductions in FPG, body weight, and systolic BP were observed with canagliflozin versus sitagliptin (P < 0.001). Overall AE rates were similar with canagliflozin (76.7%) and sitagliptin (77.5%); incidence of serious AEs and AE-related discontinuations was low for both groups. Higher incidences of genital mycotic infections and osmotic diuresis-related AEs were observed with canagliflozin, which led to one discontinuation. Hypoglycemia rates were similar in both groups.
Conclusions: Findings suggest that canagliflozin may be a new therapeutic tool providing better improvement in glycemic control and body weight reduction than sitagliptin, but with increased genital infections in subjects with type 2 diabetes using metformin plus sulfonylurea.
Trial registration: ClinicalTrials.gov NCT01137812.
Figures
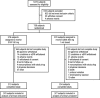
Figure 1
Study flow diagram. SITA, sitagliptin; CANA, canagliflozin.
Figure 2
Changes in efficacy parameters (last observation carried forward [LOCF]). Change in A1C (A), mean A1C over time (B), change in FPG (C), change in postprandial glucose (PPG) (D), and percent change in body weight (E). SITA, sitagliptin; CANA, canagliflozin. *Statistical comparison for CANA 300 mg versus SITA 100 mg not performed (not prespecified).
Similar articles
- Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study.
Stenlöf K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, Tong C, Canovatchel W, Meininger G. Stenlöf K, et al. Curr Med Res Opin. 2014 Feb;30(2):163-75. doi: 10.1185/03007995.2013.850066. Epub 2013 Oct 28. Curr Med Res Opin. 2014. PMID: 24073995 Clinical Trial. - Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial.
Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Lavalle-González FJ, et al. Diabetologia. 2013 Dec;56(12):2582-92. doi: 10.1007/s00125-013-3039-1. Epub 2013 Sep 13. Diabetologia. 2013. PMID: 24026211 Free PMC article. Clinical Trial. - Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone.
Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, Stein P. Forst T, et al. Diabetes Obes Metab. 2014 May;16(5):467-77. doi: 10.1111/dom.12273. Epub 2014 Mar 12. Diabetes Obes Metab. 2014. PMID: 24528605 Free PMC article. Clinical Trial. - Fixed-Dose Combination of Canagliflozin and Metformin for the Treatment of Type 2 Diabetes: An Overview.
Davidson JA, Sloan L. Davidson JA, et al. Adv Ther. 2017 Jan;34(1):41-59. doi: 10.1007/s12325-016-0434-2. Epub 2016 Nov 16. Adv Ther. 2017. PMID: 27854055 Free PMC article. Review. - Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis.
Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Yang XP, et al. Eur J Clin Pharmacol. 2014 Oct;70(10):1149-58. doi: 10.1007/s00228-014-1730-x. Epub 2014 Aug 16. Eur J Clin Pharmacol. 2014. PMID: 25124541 Review.
Cited by
- Comparison of kidney and hepatic outcomes among sodium-glucose cotransporter-2 inhibitors: a retrospective study using multiple propensity scores.
Hiura K, Suzuki C, Kubo J, Goto H, Takatori S, Ishida K, Tanaka Y, Mizutani A, Yamashita Y, Kurumazuka C, Takagi A, Kobayashi R, Shibanami A. Hiura K, et al. J Pharm Health Care Sci. 2024 Sep 17;10(1):57. doi: 10.1186/s40780-024-00378-2. J Pharm Health Care Sci. 2024. PMID: 39285495 Free PMC article. - Lessons from PROMINENT and prospects for pemafibrate.
Fruchart JC, Fruchart-Najib J, Yamashita S, Libby P, Yokote K, Kodama T, Tomita Y, Ridker PM, Hermans MP, Zambon A. Fruchart JC, et al. Cardiovasc Diabetol. 2024 Jul 29;23(1):279. doi: 10.1186/s12933-024-02305-z. Cardiovasc Diabetol. 2024. PMID: 39080716 Free PMC article. - Transporter Proteins as Therapeutic Drug Targets-With a Focus on SGLT2 Inhibitors.
Komaniecka N, Maroszek S, Drozdzik M, Oswald S, Drozdzik M. Komaniecka N, et al. Int J Mol Sci. 2024 Jun 25;25(13):6926. doi: 10.3390/ijms25136926. Int J Mol Sci. 2024. PMID: 39000033 Free PMC article. Review. - SGLT-2 Inhibitors: Potential Novel Strategy to Prevent Congestive Heart Failure in Diabetes?
Verbrugge FH, Vangoitsenhoven R, Mullens W, Van der Schueren B, Mathieu C, Tang WHW. Verbrugge FH, et al. Curr Cardiovasc Risk Rep. 2015 Aug;9(8):38. doi: 10.1007/s12170-015-0467-0. Epub 2015 Jun 17. Curr Cardiovasc Risk Rep. 2015. PMID: 38994329 Free PMC article. - Comparison of the effects of empagliflozin and sitagliptin, as add-on to metformin, on serum levels of asprosin and metabolic parameters in patients with type 2 diabetes mellitus.
Talebi SS, Rezaie S, Hajmiri MS, Zamanirafe M, Ranjbar A, Moridi H, Mirjalili M, Mehrpooya M. Talebi SS, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):9149-9165. doi: 10.1007/s00210-024-03219-z. Epub 2024 Jun 20. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38900252
References
- Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 - PMC - PubMed
- Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care 2005;28:995–1000 - PubMed
- Gross JL, Kramer CK, Leitão CB, et al. Diabetes and Endocrinology Meta-analysis Group (DEMA) Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med 2011;154:672–679 - PubMed
- National Institute for Health and Care Excellence. Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes. NICE short clinical guideline 87.2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical