Host gene expression profiling and in vivo cytokine studies to characterize the role of linezolid and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA) murine sepsis model - PubMed (original) (raw)
Host gene expression profiling and in vivo cytokine studies to characterize the role of linezolid and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA) murine sepsis model
Batu K Sharma-Kuinkel et al. PLoS One. 2013.
Abstract
Linezolid (L), a potent antibiotic for Methicillin Resistant Staphylococcus aureus (MRSA), inhibits bacterial protein synthesis. By contrast, vancomycin (V) is a cell wall active agent. Here, we used a murine sepsis model to test the hypothesis that L treatment is associated with differences in bacterial and host characteristics as compared to V. Mice were injected with S. aureus USA300, and then intravenously treated with 25 mg/kg of either L or V at 2 hours post infection (hpi). In vivo alpha-hemolysin production was reduced in both L and V-treated mice compared to untreated mice but the reduction did not reach the statistical significance [P = 0.12 for L; P = 0.70 for V). PVL was significantly reduced in L-treated mice compared to untreated mice (P = 0.02). However the reduction of in vivo PVL did not reach the statistical significance in V- treated mice compared to untreated mice (P = 0.27). Both antibiotics significantly reduced IL-1β production [P = 0.001 for L; P = 0.006 for V]. IL-6 was significantly reduced with L but not V antibiotic treatment [P<0.001 for L; P = 0.11 for V]. Neither treatment significantly reduced production of TNF-α. Whole-blood gene expression profiling showed no significant effect of L and V on uninfected mice. In S. aureus-infected mice, L altered the expression of a greater number of genes than V (95 vs. 42; P = 0.001). Pathway analysis for the differentially expressed genes identified toll-like receptor signaling pathway to be common to each S. aureus-infected comparison. Expression of immunomodulatory genes like Cxcl9, Cxcl10, Il1r2, Cd14 and Nfkbia was different among the treatment groups. Glycerolipid metabolism pathway was uniquely associated with L treatment in S. aureus infection. This study demonstrates that, as compared to V, treatment with L is associated with reduced levels of toxin production, differences in host inflammatory response, and distinct host gene expression characteristics in MRSA sepsis.
Conflict of interest statement
Competing Interests: The authors have read the journal’s policy and have the following conflicts. V.G.F. has received grants or research support from Astellas Pharma, Inc., Cubist Pharmaceuticals, Merck & Co., Inc., Theravance, Inc., MedImmune, Cerexa, Inc., Pfizer, Advanced Liquid Logic, MedImmune and the United States National Institutes of Health. He is a consultant for Astellas Pharma, Inc., Biosynexus, Novartis, Cubist Pharmaceuticals, Inimex, Merck & Co., Inc., Galderma, Johnson & Johnson, Medicines Company, Novartis and is on the speaker’s bureau and advisory committee for Cubist Pharmaceuticals. He has received honoraria from Achaogen, Arpida, Astellas Pharma, Inc., Cubist Pharmaceuticals, Durata, Inhibitex, Leo Pharma, Merck & Co., Inc., Pfizer, Targanta, Theravance, Inc., and Ortho-McNeil. This study uses Linezolid, a product produced by Pfizer, Inc. The co-author V.G.F. is a PLOS ONE Editorial Board member. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials. B.K.S.K, Y.Z., Q.Y. and S.H.A. have no competing interests to declare.
Figures
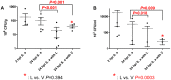
Figure 1. Effect of antibiotics on bacterial load in A/J mice.
The bacterial loads in the (A) Kidney and (B) Blood from A/J mice after intraperitoneal infection with S. aureus (6×106 CFU/g, strain USA300), and treatment with linezolid (L) or vancomycin (V) (25 mg/kg). Infected mice were sacrificed as follows: 2 hpi S. aureus; 24 hpi S. aureus; 24 hpi S. aureus with linezolid or vancomycin. Each symbol represents one mouse. _P-_values were obtained by F-test. The error bars correspond to the standard error of mean (SEM) and the dashed line correspond to the mean value. Values of P<0.05 were considered significant.
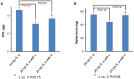
Figure 2. Effect of antibiotics on in vivo toxin production.
In vivo level of (A) Panton Valentine Leukocidin (PVL, µg) (B) and Alpha-toxin (ng) in the serum of A/J mice after 24 h of intraperitoneal (ip) infection with S. aureus (6×106 CFU/g, strain USA300), and treatment with linezolid or vancomycin (25 mg/kg) at 2 h was measured using ELISA. _P-_value was obtained by unpaired t-test. The error bar corresponds to the standard error of mean (SEM). Values of P<0.05 were considered significant.
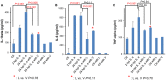
Figure 3. Effect of antibiotics on pro-inflammatory cytokines.
Level of (A) IL-1beta (pg/ml), (B) IL-6 (pg/ml), and (C)TNF-alpha (pg/ml) from the serum of A/J mice without infection, with 2 hours post infection (hpi) with S. aureus (6×106 CFU/g, strain USA300), and 24 hpi with and without antibiotic treatment as labeled was measured using ELISA. _P-_value was obtained by unpaired t-test. The error bar corresponds to the standard error of mean (SEM). Values of P<0.05 were considered significant.
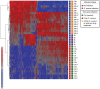
Figure 4. Unsupervised hierarchical clustering analysis of mouse blood microarray data.
Each colored row in the heat map represents the gene expression value for a probe and each column represents a sample. The color conventions are as follows: red indicates over-expressed transcripts, blue represents under-expressed transcripts. Time of analysis post-infection are marked as follows: green rectangles indicate 0 hpi, purple rectangles indicate 2 hpi, and orange rectangles indicate 24 hpi (with and without antibiotic treatment).
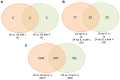
Figure 5. Comparisons of differentially expressed genes in mice with the appropriate treatment.
(A) Effect of linezolid and vancomycin on host, (B) Effect of linezolid and vancomycin on host with S. aureus infection, and (C) Effect of S. aureus infection on host immune response. Venn diagrams indicate the number of genes unique to each comparison pair and the number of genes common to each comparison pair (overlapped).
Similar articles
- Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia.
Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. Diep BA, et al. J Infect Dis. 2013 Jul;208(1):75-82. doi: 10.1093/infdis/jit129. Epub 2013 Mar 26. J Infect Dis. 2013. PMID: 23532096 Free PMC article. - Effect of linezolid on clinical severity and pulmonary cytokines in a murine model of influenza A and Staphylococcus aureus coinfection.
Liu X, He Y, Xiao K, White JR, Fusco DN, Papanicolaou GA. Liu X, et al. PLoS One. 2013;8(3):e57483. doi: 10.1371/journal.pone.0057483. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23478252 Free PMC article. - Methicillin-resistant Staphylococcus aureus-induced thrombo-inflammatory response is reduced with timely antibiotic administration.
Franks Z, Campbell RA, Vieira de Abreu A, Holloway JT, Marvin JE, Kraemer BF, Zimmerman GA, Weyrich AS, Rondina MT. Franks Z, et al. Thromb Haemost. 2013 Apr;109(4):684-95. doi: 10.1160/TH12-08-0543. Epub 2013 Jan 24. Thromb Haemost. 2013. PMID: 23348831 Free PMC article. - Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) pneumonia: linezolid or vancomycin? - Comparison of pharmacology and clinical efficacy.
Pletz MW, Burkhardt O, Welte T. Pletz MW, et al. Eur J Med Res. 2010 Nov 30;15(12):507-13. doi: 10.1186/2047-783x-15-12-507. Eur J Med Res. 2010. PMID: 21163725 Free PMC article. Review.
Cited by
- Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum.
Koon HW, Ho S, Hing TC, Cheng M, Chen X, Ichikawa Y, Kelly CP, Pothoulakis C. Koon HW, et al. Antimicrob Agents Chemother. 2014 Aug;58(8):4642-50. doi: 10.1128/AAC.02783-14. Epub 2014 Jun 2. Antimicrob Agents Chemother. 2014. PMID: 24890583 Free PMC article. - Systematic analysis of lysine crotonylation in human macrophages responding to MRSA infection.
Zhang H, Ma W, Liu H, Tang W, Shu J, Zhou J, Zheng H, Xiao H, Yang X, Liu D, Liang H, Yang X. Zhang H, et al. Front Cell Infect Microbiol. 2023 Feb 8;13:1126350. doi: 10.3389/fcimb.2023.1126350. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36844408 Free PMC article. - Subinhibitory concentrations of tedizolid potently inhibit extracellular toxin production by methicillin-sensitive and methicillin-resistant Staphylococcus aureus.
Katahira EJ, Davidson SM, Stevens DL, Bolz DD. Katahira EJ, et al. J Med Microbiol. 2019 Feb;68(2):255-262. doi: 10.1099/jmm.0.000905. Epub 2018 Dec 17. J Med Microbiol. 2019. PMID: 30556803 Free PMC article. - Review and Meta-Analyses of TAAR1 Expression in the Immune System and Cancers.
Fleischer LM, Somaiya RD, Miller GM. Fleischer LM, et al. Front Pharmacol. 2018 Jun 26;9:683. doi: 10.3389/fphar.2018.00683. eCollection 2018. Front Pharmacol. 2018. PMID: 29997511 Free PMC article. - Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections.
Thangamani S, Younis W, Seleem MN. Thangamani S, et al. Sci Rep. 2015 Jun 26;5:11596. doi: 10.1038/srep11596. Sci Rep. 2015. PMID: 26111644 Free PMC article.
References
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520–532. - PubMed
- Gould IM, David MZ, Esposito S, Garau J, Lina G, et al. (2012) New insights into meticillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents 39: 96–104. - PubMed
- Kobayashi SD, DeLeo FR (2009) An update on community-associated MRSA virulence. Curr Opin Pharmacol 9: 545–551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials