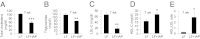Intestinal alkaline phosphatase prevents metabolic syndrome in mice - PubMed (original) (raw)
. 2013 Apr 23;110(17):7003-8.
doi: 10.1073/pnas.1220180110. Epub 2013 Apr 8.
Sulaiman R Hamarneh, Konstantinos P Economopoulos, Sayeda Nasrin Alam, Omeed Moaven, Palak Patel, Nondita S Malo, Madhury Ray, Seyed M Abtahi, Nur Muhammad, Atri Raychowdhury, Abeba Teshager, Mussa M Rafat Mohamed, Angela K Moss, Rizwan Ahmed, Shahrad Hakimian, Sonoko Narisawa, José Luis Millán, Elizabeth Hohmann, H Shaw Warren, Atul K Bhan, Madhu S Malo, Richard A Hodin
Affiliations
- PMID: 23569246
- PMCID: PMC3637741
- DOI: 10.1073/pnas.1220180110
Intestinal alkaline phosphatase prevents metabolic syndrome in mice
Kanakaraju Kaliannan et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Metabolic syndrome comprises a cluster of related disorders that includes obesity, glucose intolerance, insulin resistance, dyslipidemia, and fatty liver. Recently, gut-derived chronic endotoxemia has been identified as a primary mediator for triggering the low-grade inflammation responsible for the development of metabolic syndrome. In the present study we examined the role of the small intestinal brush-border enzyme, intestinal alkaline phosphatase (IAP), in preventing a high-fat-diet-induced metabolic syndrome in mice. We found that both endogenous and orally supplemented IAP inhibits absorption of endotoxin (lipopolysaccharides) that occurs with dietary fat, and oral IAP supplementation prevents as well as reverses metabolic syndrome. Furthermore, IAP supplementation improves the lipid profile in mice fed a standard, low-fat chow diet. These results point to a potentially unique therapy against metabolic syndrome in at-risk humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
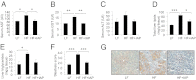
Fig. 1.
IAP prevents endotoxemia. Groups of 4- to 17-wk-old C57BL/6 male IAP-KO (_Akp3_−/−) mice and their WT littermates (n = 5–10 each group) were used in these experiments unless otherwise indicated (
SI Materials and Methods
). Mice received a low-fat diet (LFD, 14% kcal from fat) unless otherwise indicated. (A) Serum endotoxin levels. (B) Serum TNF-α levels. (C) Intestinal permeability as determined by quantifying the amount of FITC-dextran (70 kDa) levels in the serum after its oral gavage. (D) Change in serum endotoxin levels after oral LPS gavage. (E) Blood glucose levels during glucose tolerance test (GTT). (F) Serum insulin levels during the first 30 min of GTT. (G) Blood glucose levels during insulin tolerance test (ITT). (H) Body weight gain by mice on LFD. (I) White adipose tissue (WAT) distribution. (J) Blood glucose levels during GTT in mice receiving LFD (LF group) or high-fat diet (HFD, 45% kcal from fat) (HF group) treated with
l
-phenylalanine (Phe, 10 mM in the drinking water), a specific inhibitor of IAP. (K) Inhibition of corn-oil–induced endotoxemia in CD-1 mice by IAP in a dose-dependent manner. (L) Inhibition of corn-oil–induced endotoxemia in WT and IAP-KO mice. (M) Inhibition of corn-oil– plus LPS-induced endotoxemia in CD-1 mice by IAP in a dose-dependent manner. Statistics: data expressed as mean ± SEM. Two-tailed unpaired Student’s t test. For multiple comparisons, analysis of variance with Tukey was used. * or #P < 0.05; ** or ##P < 0.01; *** or ###P < 0.001. The number sign (#) refers to the HF vs. HF + Phe comparison.
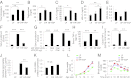
Fig. 2.
IAP prevents chronic HFD-induced glucose intolerance and insulin resistance. Groups of 15-wk-old WT male C57BL/6 mice (n = 5 for each group) were fed an HFD (45% kcal from fat) ± IAP (100 units/mL in drinking water) for 11 wk. A control group of mice received LFD (14% kcal from fat). (A) Blood glucose levels during GTT in mice receiving LFD (LF group), HFD (HF group), and HFD + IAP (HF + IAP group). (B) Serum insulin levels during GTT. (C) Insulin resistance index (homeostasis model assessment of insulin resistance, HOMA-IR). (D) Blood glucose levels during ITT. (E) Weight of pancreas. (F) White adipose tissue (WAT) expressed as percentage of body weight (BW). (G) Adiposity index. (H) Energy intake by different groups. Statistics: data expressed as mean ± SEM. Two-tailed unpaired Student’s t test. For multiple comparisons, analysis of variance with Tukey was used. * or #P < 0.05; ** or ##P < 0.01; *** or ###P < 0.001. The asterisk (*) refers to the LF vs. HF comparison and the number sign (#) refers to the HF vs. HF + IAP comparison.
Fig. 3.
IAP prevents HFD-induced liver injury. (A) Serum aspartate aminotransferase (AST) levels (see Fig. 2 for description of mice). (B) Serum gamma-glutamyl transferase (GGT) levels. (C) Serum alanine aminotransferase (ALT) levels. (D) Total liver lipids. (E) Liver triglyceride levels. (F) Liver steatosis score. (G) Oil Red O staining of frozen liver sections (10× objective). Statistics: data expressed as mean ± SEM. Two-tailed unpaired Student’s t test. For multiple comparisons, analysis of variance with Tukey was used. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 4.
IAP prevents HFD-induced dyslipidemia and endotoxemia. (A) Serum total cholesterol levels (see Fig. 2 for description of mice). (B) Levels of serum triglycerides. (C) Serum low-density lipoprotein cholesterol (LDL-C) levels. (D) Serum high-density lipoprotein cholesterol (HDL-C) levels. (E) Ratio between HDL-C and LDL-C. (F) Serum endotoxin levels. (G) Serum endotoxin levels after i.p. injection of LPS (different groups of mice, see
SI Materials and Methods
). (H) Serum tumor necrosis factor-alpha (TNF-α) levels. (I) Cecal TNF-α levels. (J) Intestinal permeability as determined by quantifying the amount of FITC-dextran (70 kDa) levels in the serum after its oral gavage (different groups of mice, see
SI Materials and Methods
). (K) Relative expression of duodenal IAP (Akp3) mRNA as determined by qRT-PCR (different groups of mice, see
SI Materials and Methods
). (L) Body weight after 4 wk of HFD feeding, which was preceded by 3 wk of oral antibiotics treatment [ampicillin (1 g/L) plus norfloxacin (1 g/L)] (see
Fig. S4_P_
for energy intake) (different groups of mice, see
SI Materials and Methods
). (M) GTT after 4 wk of HFD feeding (see
Fig. S4_Q_
for AUC) (see L for description of mice). Statistics: data expressed as mean ± SEM. Two-tailed unpaired Student’s t test. For multiple comparisons, analysis of variance with Tukey was used. *P < 0.05; **P < 0.01; ***P < 0.001. The asterisk (*) refers to the LF vs. HF comparison, and no statistically significant difference was observed between HF and HF + IAP groups (for M).
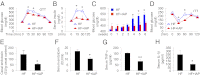
Fig. 5.
IAP cures HFD-induced metabolic syndrome. Groups of 5-wk-old WT male C57BL/6 mice (n = 5 for each group) were fed HFD (45% kcal from fat) for 14 wk to induce metabolic syndrome (
Fig. S5
). Mice with metabolic syndrome were then treated with or without IAP (100 units/mL drinking water) for 6 wk. (A) Blood glucose levels during GTT. (B) Serum insulin levels during GTT. (C) Weekly blood glucose levels during GTT at 15-min time point. (D) Blood glucose levels during ITT. (E) Cecal endotoxin levels. (F) Serum endotoxin levels. (G) Serum TNF-α levels. (H) Serum IL-1β levels. Statistics: data expressed as mean ± SEM. Two-tailed unpaired Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 6.
Low-fat diet (LFD) supplemented with IAP improves lipid profile. Groups of 5-wk-old WT female C57BL/6 mice (n = 5) were fed LFD (14% kcal from fat) ± IAP (100 units/mL drinking water) for 7 wk. (A) Serum total cholesterol levels. (B) Serum triglyceride levels. (C) Serum LDL-C levels. (D) Serum HDL-C levels. (E) Ratio between HDL-C and LDL-C. Statistics: data expressed as mean ± SEM. Two-tailed unpaired Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001.
Comment in
- Inflammation: Intestinal-type alkaline phosphatase--a new therapeutic weapon?
Koch L. Koch L. Nat Rev Endocrinol. 2013 Jul;9(7):379. doi: 10.1038/nrendo.2013.95. Epub 2013 Apr 23. Nat Rev Endocrinol. 2013. PMID: 23609332 No abstract available.
Similar articles
- Intestine-specific expression of human chimeric intestinal alkaline phosphatase attenuates Western diet-induced barrier dysfunction and glucose intolerance.
Ghosh SS, He H, Wang J, Korzun W, Yannie PJ, Ghosh S. Ghosh SS, et al. Physiol Rep. 2018 Jul;6(14):e13790. doi: 10.14814/phy2.13790. Physiol Rep. 2018. PMID: 30058275 Free PMC article. - Loss of Intestinal Alkaline Phosphatase Leads to Distinct Chronic Changes in Bone Phenotype.
Kuehn F, Adiliaghdam F, Hamarneh SR, Vasan R, Liu E, Liu Y, Ramirez JM, Hoda RS, Munoz AR, Ko FC, Armanini M, Brooks DJ, Bouxsein ML, Demay MB, Hodin RA. Kuehn F, et al. J Surg Res. 2018 Dec;232:325-331. doi: 10.1016/j.jss.2018.06.061. Epub 2018 Jul 18. J Surg Res. 2018. PMID: 30463736 - Inhibition of the gut enzyme intestinal alkaline phosphatase may explain how aspartame promotes glucose intolerance and obesity in mice.
Gul SS, Hamilton AR, Munoz AR, Phupitakphol T, Liu W, Hyoju SK, Economopoulos KP, Morrison S, Hu D, Zhang W, Gharedaghi MH, Huo H, Hamarneh SR, Hodin RA. Gul SS, et al. Appl Physiol Nutr Metab. 2017 Jan;42(1):77-83. doi: 10.1139/apnm-2016-0346. Epub 2016 Nov 18. Appl Physiol Nutr Metab. 2017. PMID: 27997218 Free PMC article. - Multisystemic functions of alkaline phosphatases.
Buchet R, Millán JL, Magne D. Buchet R, et al. Methods Mol Biol. 2013;1053:27-51. doi: 10.1007/978-1-62703-562-0_3. Methods Mol Biol. 2013. PMID: 23860646 Review. - Role of Intestinal Alkaline Phosphatase in Innate Immunity.
Singh SB, Lin HC. Singh SB, et al. Biomolecules. 2021 Nov 29;11(12):1784. doi: 10.3390/biom11121784. Biomolecules. 2021. PMID: 34944428 Free PMC article. Review.
Cited by
- Duodenal chemosensory system: enterocytes, enteroendocrine cells, and tuft cells.
Akiba Y, Hashimoto S, Kaunitz JD. Akiba Y, et al. Curr Opin Gastroenterol. 2020 Nov;36(6):501-508. doi: 10.1097/MOG.0000000000000685. Curr Opin Gastroenterol. 2020. PMID: 32925177 Free PMC article. Review. - Alkaline phosphatase: a potential biomarker for stroke and implications for treatment.
Brichacek AL, Brown CM. Brichacek AL, et al. Metab Brain Dis. 2019 Feb;34(1):3-19. doi: 10.1007/s11011-018-0322-3. Epub 2018 Oct 4. Metab Brain Dis. 2019. PMID: 30284677 Free PMC article. Review. - TNAP: A New Multitask Enzyme in Energy Metabolism.
Briolay A, Bessueille L, Magne D. Briolay A, et al. Int J Mol Sci. 2021 Sep 28;22(19):10470. doi: 10.3390/ijms221910470. Int J Mol Sci. 2021. PMID: 34638808 Free PMC article. Review. - Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease.
Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Kozlitina J, et al. Nat Genet. 2014 Apr;46(4):352-6. doi: 10.1038/ng.2901. Epub 2014 Feb 16. Nat Genet. 2014. PMID: 24531328 Free PMC article. - Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates.
Malo MS, Moaven O, Muhammad N, Biswas B, Alam SN, Economopoulos KP, Gul SS, Hamarneh SR, Malo NS, Teshager A, Mohamed MM, Tao Q, Narisawa S, Millán JL, Hohmann EL, Warren HS, Robson SC, Hodin RA. Malo MS, et al. Am J Physiol Gastrointest Liver Physiol. 2014 May 15;306(10):G826-38. doi: 10.1152/ajpgi.00357.2013. Epub 2014 Apr 10. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24722905 Free PMC article.
References
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association National Heart, Lung, and Blood Institute Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. - PubMed
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. - PubMed
- Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28(11):2745–2749. - PubMed
- Malik S, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. - PubMed
- Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DK050623/DK/NIDDK NIH HHS/United States
- R01 DK047186/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- R01 DE012889/DE/NIDCR NIH HHS/United States
- 2P30 DK43351/DK/NIDDK NIH HHS/United States
- R01 DK050623/DK/NIDDK NIH HHS/United States
- R01DK047186/DK/NIDDK NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R01 AR053102/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials