Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4 - PubMed (original) (raw)
Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4
Shigeru Shibata et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Pseudohypoaldosteronism type II (PHAII) is a rare Mendelian syndrome featuring hypertension and hyperkalemia resulting from constitutive renal salt reabsorption and impaired K(+) secretion. Recently, mutations in Kelch-like 3 (KLHL3) and Cullin 3 (CUL3), components of an E3 ubiquitin ligase complex, were found to cause PHAII, suggesting that loss of this complex's ability to target specific substrates for ubiquitination leads to PHAII. By MS and coimmunoprecipitation, we show that KLHL3 normally binds to WNK1 and WNK4, members of WNK (with no lysine) kinase family that have previously been found mutated in PHAII. We show that this binding leads to ubiquitination, including polyubiquitination, of at least 15 specific sites in WNK4, resulting in reduced WNK4 levels. Dominant disease-causing mutations in KLHL3 and WNK4 both impair WNK4 binding, ubiquitination, and degradation. WNK4 normally induces clearance of the renal outer medullary K(+) channel (ROMK) from the cell surface. We show that WT but not mutant KLHL3 inhibits WNK4-induced reduction of ROMK level. We show that PHAII-causing mutations in WNK4 lead to a marked increase in WNK4 protein levels in the kidney in vivo. These findings demonstrate that CUL3-RING (really interesting new gene) ligases that contain KLHL3 target ubiquitination of WNK4 and thereby regulate WNK4 levels, which in turn regulate levels of ROMK. These findings reveal a specific role of CUL3 and KLHL3 in electrolyte homeostasis and provide a molecular explanation for the effects of disease-causing mutations in both KLHL3 and WNK4.
Keywords: Gordon syndrome; Kir1.1; proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
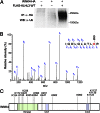
Association of WNK kinases with CUL3–KLHL3 complex. (A and B) KLHL3 binds to endogenous CUL3 and WNK1. Products of IP of FLAG-KLHL3 from COS-7 cell lysates were analyzed by LC-MS/MS. Representative MS/MS spectra from peptides mapping to CUL3 (Macaca mulatta, amino acids 717–731) (A) or WNK1 (Macaca mulatta, amino acids 1855–1868) (B) are shown (all peptides in
Tables S1
and
S2
). (C) IP of cell lysates with anti-FLAG (KLHL3) was followed by Western blotting (WB). CUL3 is pulled down only when FLAG-KLHL3 is expressed. (D) Cell lysates expressing indicated proteins were immunoprecipitated with anti-HA, followed by Western blotting. When WNK4-HA is coexpressed with KLHL3, both KLHL3 and CUL3 are immunoprecipitated, whereas neither CUL3 nor KLHL3 is detected in IP products in the absence of KLHL3. (E) Cell lysates expressing the indicated proteins were immunoprecipitated using FLAG antibody to IP FLAG-KLHL3, followed by Western analysis with antibody to HA to detect WNK4-HA (Upper) or FLAG to detect KLHL3 (Lower). IP of FLAG-KLHL3 pulls down WNK4.
Fig. 2.
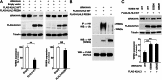
PHAII mutations in WNK4 or KLHL3 abrogate WNK4 binding to KLHL3. Cell lysates from cells expressing FLAG-KLHL3WT or FLAG-KLHL3R528H were incubated with lysates from cells expressing WNK4WT-HA, WNK4E559K-HA, or WNK4Q562E-HA followed by anti-FLAG (KLHL3) immunoprecipitation and Western blotting (WB). PHAII mutations in KLHL3 and WNK4 eliminate or reduce binding of WNK4 to KLHL3.
Fig. 3.
Identification of ubiquitin conjugation sites in WNK4 by MS. (A) Cell lysates expressing the indicated proteins were immunoprecipitated in denaturing condition with anti-HA, followed by Western blotting (WB) with anti-ubiquitin antibodies. WNK4 is ubiquitinated in the presence but not the absence of KLHL3. (B) MS/MS spectrum of purified ubiquitinated WNK4 showing assignment of the peptide containing ubiquitinated K325 in the WNK4 kinase domain. The precursor ion, 635.882+, was selected and produced the fragment ion spectrum shown. Specific y and b fragment ions allowed the identification of K325 with di-glycine modification (shown as GG) that is derived from the C terminus of ubiquitin. (C) Ubiquitination sites of WNK4 in COS-7 cells expressing KLHL3. Sites are numbered according to their position in mouse sequence. The domains of WNK4 are indicated and lysines that are ubiquitinated are shown. Peptides and Mascot scores are shown in Table 1. CC, coiled-coil domain.
Fig. 4.
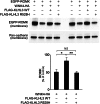
KLHL3WT, but not KLHL3R528H reduces WNK4 levels. (A) Western blots of cell lysates show markedly reduced levels of WNK4 when KLHL3WT but not KLHL3R528H is coexpressed. An empty vector was used as control to equalize the total amount of DNA in transfections lacking KLHL3. (Lower) Bar graphs show quantitation of the results of four independent experiments in each condition. **P < 0.01. (B) COS-7 cell lysates expressing the indicated proteins were immunoprecipitated with anti-HA, followed by Western blotting (WB) with anti-ubiquitin antibodies. Polyubiquitination of WNK4 was markedly reduced in the presence of KLHL3R528H. In addition, WNK4 level was reduced in cells expressing KLHL3WT compared with KLHL3R528H. Representative results of triplicate experiments are shown. (C) Western blots of lysates of cells expressing FLAG-KLHL3WT and either WNK4WT-HA, WNK4E559K-HA, or WNK4Q562E-HA are shown. Mutant WNK4s show significantly highly levels compared with WT. Bar graph shows the results of quantitation (n = 4). Data are expressed as mean ± SEM.
Fig. 5.
KLHL3 abrogates WNK4’s inhibition of ROMK expression. The indicated proteins were expressed, and levels of EGFP-ROMK in the membrane fraction were analyzed by Western blotting (Upper). Cadherin was used as a loading control (Lower). KLHL3WT inhibits WNK4-dependent reduction of ROMK level, and effect lost with KLHL3R528H. Results from biological replicates are shown and bar graphs show the results of quantitation (n = 4). Data are expressed as means ± SEM; *P < 0.05, **P < 0.01.
Fig. 6.
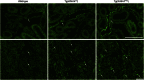
Renal expression of WNK4 in WT, Tg(WNK4WT), and Tg(WNK4PHAII) mice. Kidneys from 3- to 5-mo-old mice of indicated genotypes (n = 3 of each) were fixed by in vivo perfusion and sectioned and stained with anti-WNK4. (Upper) High-power and (Lower) low-power images. Intensity of WNK4 staining (arrows) was consistently increased in Tg(WNK4PHAII) mice compared with WT littermates or Tg(WNK4WT). (Scale bars, 100 μm.)
Comment in
- Control of electrolyte balance through ubiquitination.
Herrera M, Coffman TM. Herrera M, et al. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7535-6. doi: 10.1073/pnas.1305242110. Epub 2013 Apr 23. Proc Natl Acad Sci U S A. 2013. PMID: 23613582 Free PMC article. No abstract available.
Similar articles
- The CUL3-KLHL3 E3 ligase complex mutated in Gordon's hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction.
Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. Ohta A, et al. Biochem J. 2013 Apr 1;451(1):111-22. doi: 10.1042/BJ20121903. Biochem J. 2013. PMID: 23387299 Free PMC article. - Kelch-like 3/Cullin 3 ubiquitin ligase complex and WNK signaling in salt-sensitive hypertension and electrolyte disorder.
Sohara E, Uchida S. Sohara E, et al. Nephrol Dial Transplant. 2016 Sep;31(9):1417-24. doi: 10.1093/ndt/gfv259. Epub 2015 Jul 6. Nephrol Dial Transplant. 2016. PMID: 26152401 Review. - Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice.
Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, Isobe K, Inoue Y, Takeishi K, Takeda N, Sasaki S, Uchida S. Susa K, et al. Hum Mol Genet. 2014 Oct 1;23(19):5052-60. doi: 10.1093/hmg/ddu217. Epub 2014 May 12. Hum Mol Genet. 2014. PMID: 24821705 - Decrease of WNK4 ubiquitination by disease-causing mutations of KLHL3 through different molecular mechanisms.
Mori Y, Wakabayashi M, Mori T, Araki Y, Sohara E, Rai T, Sasaki S, Uchida S. Mori Y, et al. Biochem Biophys Res Commun. 2013 Sep 13;439(1):30-4. doi: 10.1016/j.bbrc.2013.08.035. Epub 2013 Aug 17. Biochem Biophys Res Commun. 2013. PMID: 23962426 - Regulation of blood pressure and renal electrolyte balance by Cullin-RING ligases.
Uchida S. Uchida S. Curr Opin Nephrol Hypertens. 2014 Sep;23(5):487-93. doi: 10.1097/MNH.0000000000000049. Curr Opin Nephrol Hypertens. 2014. PMID: 24992566 Review.
Cited by
- COP9 signalosome deletion promotes renal injury and distal convoluted tubule remodeling.
Cornelius RJ, Nelson JW, Su XT, Yang CL, Ellison DH. Cornelius RJ, et al. Am J Physiol Renal Physiol. 2022 Jul 1;323(1):F4-F19. doi: 10.1152/ajprenal.00436.2021. Epub 2022 May 9. Am J Physiol Renal Physiol. 2022. PMID: 35532068 Free PMC article. - The Less Well-Known Little Brothers: The SLC9B/NHA Sodium Proton Exchanger Subfamily-Structure, Function, Regulation and Potential Drug-Target Approaches.
Anderegg MA, Gyimesi G, Ho TM, Hediger MA, Fuster DG. Anderegg MA, et al. Front Physiol. 2022 May 25;13:898508. doi: 10.3389/fphys.2022.898508. eCollection 2022. Front Physiol. 2022. PMID: 35694410 Free PMC article. Review. - Inwardly rectifying K+ channels 4.1 and 5.1 (Kir4.1/Kir5.1) in the renal distal nephron.
Wang WH, Lin DH. Wang WH, et al. Am J Physiol Cell Physiol. 2022 Aug 1;323(2):C277-C288. doi: 10.1152/ajpcell.00096.2022. Epub 2022 Jun 27. Am J Physiol Cell Physiol. 2022. PMID: 35759440 Free PMC article. Review. - Overexpression of WNK1 in POMC-expressing neurons reduces weigh gain via WNK4-mediated degradation of Kir6.2.
Chung WY, Han JW, Heo W, Lee MG, Kim JY. Chung WY, et al. Mol Cell Biochem. 2018 Oct;447(1-2):165-174. doi: 10.1007/s11010-018-3301-4. Epub 2018 Feb 1. Mol Cell Biochem. 2018. PMID: 29392534 - Mutant Cullin 3 causes familial hyperkalemic hypertension via dominant effects.
Ferdaus MZ, Miller LN, Agbor LN, Saritas T, Singer JD, Sigmund CD, McCormick JA. Ferdaus MZ, et al. JCI Insight. 2017 Dec 21;2(24):e96700. doi: 10.1172/jci.insight.96700. JCI Insight. 2017. PMID: 29263298 Free PMC article.
References
- Lifton RP, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355(6357):262–265. - PubMed
- Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008;70:329–355. - PubMed
- Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293(5532):1107–1112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30-DA018343/DA/NIDA NIH HHS/United States
- P30 DK079310/DK/NIDDK NIH HHS/United States
- P30-DK079310/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 DA018343/DA/NIDA NIH HHS/United States
- UL1-RR024139/RR/NCRR NIH HHS/United States
- P01 DK017433/DK/NIDDK NIH HHS/United States
- P01-DK017433/DK/NIDDK NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases