Latency-associated degradation of the MRP1 drug transporter during latent human cytomegalovirus infection - PubMed (original) (raw)
Latency-associated degradation of the MRP1 drug transporter during latent human cytomegalovirus infection
Michael P Weekes et al. Science. 2013.
Abstract
The reactivation of latent human cytomegalovirus (HCMV) infection after transplantation is associated with high morbidity and mortality. In vivo, myeloid cells and their progenitors are an important site of HCMV latency, whose establishment and/or maintenance require expression of the viral transcript UL138. Using stable isotope labeling by amino acids in cell culture-based mass spectrometry, we found a dramatic UL138-mediated loss of cell surface multidrug resistance-associated protein-1 (MRP1) and the reduction of substrate export by this transporter. Latency-associated loss of MRP1 and accumulation of the cytotoxic drug vincristine, an MRP1 substrate, depleted virus from naturally latent CD14(+) and CD34(+) progenitors, all of which are in vivo sites of latency. The UL138-mediated loss of MRP1 provides a marker for detecting latent HCMV infection and a therapeutic target for eliminating latently infected cells before transplantation.
Figures
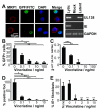
Fig. 1. HCMV UL138 downregulates cell surface MRP1 and other targets
(A) Scatterplot of proteins identified in PMP and quantified by >2 unique peptides. The summed ion intensity (y-axis) is shown as log10. Significance A was used to estimate p-values (28). (B) Cytofluorometric analysis of the indicated proteins in THP-1 cells stably expressing HCMV-encoded UL138 (THP-UL138) and control THP-1 cells. (C/D) Immunoblot for MRP1/UL138 in control or UL138-transduced fibroblasts (C), or UL138-transduced THP-1 cells (D).
Fig. 2. UL138 downregulates MRP1 during productive HCMV infection
(A) Mock or TB40 IE2-eYFP infected HFFs (m.o.i. 5). At 24 and 48h post-infection cells were harvested, and analysed by immunoblot. (B) Mock, Toledo wt, Toledo ΔUL133-138 or Toledo ΔUL138 virus infected HFFs were analysed by immunoblot 48h post infection (top 3 panels). UL138 and IE mRNA was analysed (RT-PCR) with GAPDH (internal mRNA control) (bottom 3 panels). (C) Differentiated primary monocytes were infected with wtTB40 or TB40ΔUL138. 72h post infection, cells were stained for MRP1, IE and DAPI prior to confocal microscopy.
Fig. 3. UL138 targets mature MRP1 for lysosomal degradation, and inhibits export of MRP1-specific substrates
(A) THP-1 or THP-UL138 cells were loaded with SNARF-1 ester and intracellular SNARF-1 measured by cytofluorometry. The proportion of cells retaining SNARF-1 were plotted. (B) 15h post infection, HCMV-infected HFFs (m.o.i 5) were analysed for intracellular SNARF-1 (left panel) and immunoblot (right panel). Three independent replicates were used per time point. Plotted: mean+/−SEM, relative to the post-load HCMV-infected sample. Two-tailed p-values (*p<0.05). (C) LTC4 export assayed in A23187-stimulated cells (Fig. S3) (28), with three independent replicates per condition. Plotted: mean+/−SEM and two-tailed p-values (*p<1×10−6,**p<0.0005). (D) RT-qPCR analysis of MRP1 and GAPDH (28). (E) MRP1 immunoprecipitations (QCRL3 antibody) from cells radiolabeled and chased as indicated, with CcmA included at the 5 hour time point (5*). Total MRP1 is quantified as percentage of MRP1 at time 0. (F) Cells incubated with MG132, CcmA, or DMSO (28) and immunoblotted as indicated. (G) HA immunoprecipitation from ADR-UL138HA cells pre-incubated with CcmA for 24 hours to increase MRP1 expression. Anti-FLAG beads were used as a control.
Fig. 4. Selective vincristine-mediated depletion of HCMV-infected cells from experimental and natural latent infection
(A) CD34+ progenitors were latently infected with TB40gfp. 72h post-infection, cells were examined (28) by confocal microscopy (left panels). GFP signal in latently-infected cells was boosted with anti-GFP FITC antibody (28). RT-PCR confirmed latent infection (right panel). (B - C) Treatment of experimentally latently-infected monocytes with vincristine reduced latent viral load as determined by detection of latently-expressed UL138 mRNA by RT-qPCR, and the relative number of latently-infected cells. Primary CD14+ monocytes were latently infected with TB40gfp. After 3 days, vincristine was added at the indicated concentration (28). 4 days later, the GFP+ cells were counted in 5 independent replicates. 3 further independent replicates were analysed by RT-qPCR for UL138, IE and GAPDH (C). IE RT-qPCR was always below the limit of detection. Plotted: mean+/−SEM and two-tailed p-values (*p<0.005,**p<0.001,***p<0.05). (D) Primary CD14+ monocytes from HCMV-seropositive donor D were treated for 4 days with vincristine (28). Endogenous HCMV was reactivated by differentiation and maturation to mature DC (22), cocultured with fibroblasts for 2 weeks (4 replicates/condition) which were examined for viral IE protein, and foci counted. Plotted are mean+/−SEM %IE+ foci compared to 0ng/ml vincristine and two-tailed p-values (*p<0.005,**p<0.001,***p<0.0005). (E) Primary CD34+ progenitors were treated for 4 days with vincristine (28). Endogenous HCMV was reactivated by differentiation and maturation to mature DC (22) then cocultured with fibroblasts for 2 weeks. Cell supernatants were transferred onto fresh fibroblasts (8 replicates/condition) and 100 cells/replicate examined after 4 days for viral IE protein with DNA counterstaining. Plotted: mean+/−SEM and two-tailed p-values (each treatment vs 0ng/ml vincristine):*p<5×10−8,**p<5×10−9.
Similar articles
- Human cytomegalovirus manipulation of latently infected cells.
Sinclair JH, Reeves MB. Sinclair JH, et al. Viruses. 2013 Nov 21;5(11):2803-24. doi: 10.3390/v5112803. Viruses. 2013. PMID: 24284875 Free PMC article. Review. - Latency-Associated Expression of Human Cytomegalovirus US28 Attenuates Cell Signaling Pathways To Maintain Latent Infection.
Krishna BA, Poole EL, Jackson SE, Smit MJ, Wills MR, Sinclair JH. Krishna BA, et al. mBio. 2017 Dec 5;8(6):e01754-17. doi: 10.1128/mBio.01754-17. mBio. 2017. PMID: 29208743 Free PMC article. - The Golgi sorting motifs of human cytomegalovirus UL138 are not required for latency maintenance.
Gelbmann CB, Kalejta RF. Gelbmann CB, et al. Virus Res. 2019 Sep;270:197646. doi: 10.1016/j.virusres.2019.197646. Epub 2019 Jun 28. Virus Res. 2019. PMID: 31260705 Free PMC article. - The Role of the Human Cytomegalovirus UL133-UL138 Gene Locus in Latency and Reactivation.
Mlera L, Moy M, Maness K, Tran LN, Goodrum FD. Mlera L, et al. Viruses. 2020 Jul 1;12(7):714. doi: 10.3390/v12070714. Viruses. 2020. PMID: 32630219 Free PMC article. Review.
Cited by
- Circulating dendritic cells isolated from healthy seropositive donors are sites of human cytomegalovirus reactivation in vivo.
Reeves MB, Sinclair JH. Reeves MB, et al. J Virol. 2013 Oct;87(19):10660-7. doi: 10.1128/JVI.01539-13. Epub 2013 Jul 24. J Virol. 2013. PMID: 23885077 Free PMC article. - Transient activation of human cytomegalovirus lytic gene expression during latency allows cytotoxic T cell killing of latently infected cells.
Krishna BA, Lau B, Jackson SE, Wills MR, Sinclair JH, Poole E. Krishna BA, et al. Sci Rep. 2016 Apr 19;6:24674. doi: 10.1038/srep24674. Sci Rep. 2016. PMID: 27091512 Free PMC article. - Selective modulation of cell surface proteins during vaccinia infection: A resource for identifying viral immune evasion strategies.
Depierreux DM, Altenburg AF, Soday L, Fletcher-Etherington A, Antrobus R, Ferguson BJ, Weekes MP, Smith GL. Depierreux DM, et al. PLoS Pathog. 2022 Jun 21;18(6):e1010612. doi: 10.1371/journal.ppat.1010612. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35727847 Free PMC article. - Human cytomegalovirus manipulation of latently infected cells.
Sinclair JH, Reeves MB. Sinclair JH, et al. Viruses. 2013 Nov 21;5(11):2803-24. doi: 10.3390/v5112803. Viruses. 2013. PMID: 24284875 Free PMC article. Review. - Mitogen and stress activated kinases act co-operatively with CREB during the induction of human cytomegalovirus immediate-early gene expression from latency.
Kew VG, Yuan J, Meier J, Reeves MB. Kew VG, et al. PLoS Pathog. 2014 Jun 12;10(6):e1004195. doi: 10.1371/journal.ppat.1004195. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24945302 Free PMC article.
References
- Mocarski ES, Shenk T, Pass RF, editors. Cytomegaloviruses. ed. 5 Vol. 5. Lipincott Williams and Wilkins; Philadelphia: 2007. pp. 2701–2773.
- Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J.Infect.Dis. 2002;185:273. - PubMed
- Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J.Gen.Virol. 1991;72:2059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0701279/MRC_/Medical Research Council/United Kingdom
- 084957/WT_/Wellcome Trust/United Kingdom
- 093966/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 100140/WT_/Wellcome Trust/United Kingdom
- 084957/Z/08/Z/WT_/Wellcome Trust/United Kingdom
- 093966/WT_/Wellcome Trust/United Kingdom
- MR/K021087/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials