Blockade of chronic type I interferon signaling to control persistent LCMV infection - PubMed (original) (raw)
Blockade of chronic type I interferon signaling to control persistent LCMV infection
Elizabeth B Wilson et al. Science. 2013.
Abstract
Type I interferons (IFN-I) are critical for antiviral immunity; however, chronic IFN-I signaling is associated with hyperimmune activation and disease progression in persistent infections. We demonstrated in mice that blockade of IFN-I signaling diminished chronic immune activation and immune suppression, restored lymphoid tissue architecture, and increased immune parameters associated with control of virus replication, ultimately facilitating clearance of the persistent infection. The accelerated control of persistent infection induced by blocking IFN-I signaling required CD4 T cells and was associated with enhanced IFN-γ production. Thus, we demonstrated that interfering with chronic IFN-I signaling during persistent infection redirects the immune environment to enable control of infection.
Figures
Fig. 1. Prolonged IFN-I signaling during persistent infection
(A) Splenic virus titers ± SD after acute LCMV-Arm (black) and persistent LCMV-Cl13 (red) infection (left). Gene expression kinetics by microarray analysis of the indicated IFNR-inducible, STAT, and IFN-I regulatory factors (IRFs) in whole spleen tissue after LCMV-Arm (black) or LCMV-Cl13 (red) relative to naïve (uninfected) mice. Each value indicates the average of three to four mice per group per time point. Values are shown without error bars for clarity, and the P values for each gene are indicated in table S1. PFU, plaque-forming units. (B) _IFN_α (left) and _IFN_β (right) mRNA expression relative to a control gene, HPRT, in splenic DCs from naïve or LCMV-Arm– or LCMV-Cl13–infected mice (day 9). N.D. indicates that _IFN_α or _IFN_β transcripts were not detected after 40 cycles of amplification. HPRT mRNA expression was measurable in all samples. (C) Mx1, OAS, and IRF3 mRNA expression relative to HPRT in the indicated IL-10+ (GFP+, green) or IL-10− (GFP−, black) splenic DCs (top) or macrophages (bottom) 9 days after LCMV-Cl13 infection of Vert-X IL-10 reporter mice. For (B) and (C), each group is a pool of cells from six to eight mice and is representative of two independent experiments.
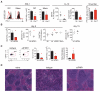
Fig. 2. The immunosuppressive program during persistent infection is dependent on IFN-I signaling
(A) Representative histograms and summarized quantification of geometric mean fluorescence intensity (GMFI) show PD-L1 on splenic DCs and macrophages 9 days after LCMV-Cl13 infection in wild-type (WT, black) and _Ifnar1_−/− (red) mice. Scatter plots show plasma IL-10 levels on day 9 after LCMV-Cl13 infection. Bar graphs measure IL-10 production by cultured splenocytes (in the absence of exogenous stimulation) isolated 9 days after LCMV-Cl13 infection from WT or _Ifnar1_−/− mice. Plasma viral titers in WT or _Ifnar1_−/− mice on day 9 after LCMV-Cl13 infection are shown to the far right. Dashed lines indicate the level of detection of the plaque assay (200 PFU). (B to D) WT mice were treated with isotype or IFNR1 blocking antibody beginning 1 day before LCMV-Cl13 infection. (B) Graphs indicate GMFI of PD-L1 on splenic CD45+ cells (left), plasma levels of IL-10 (middle), and plasma viral titers (right) of untreated mice (B6), isotype-treated mice, IFNR1 blocking antibody (αIFNR1)–treated mice, and untreated _Ifnar1_−/− mice. Plasma IL-10 levels on day 15 after LCMV-Cl13 infection are shown on the far right. (C) Flow cytometry plots of IL-10 reporter expression (GFP) in splenic DCs from Vert-X mice treated with isotype or IFNR1 blocking antibody. Bar graphs show the frequency of IL-10 expression and the GMFI of PD-L1 expression by splenic DCs. The ratio of IL-10–nonproducing to IL-10–producing DCs is shown on the far right. (D) Hematoxylin and eosin staining of spleens from naïve mice (left) or on day 9 after LCMV-Cl13 infection of mice treated with isotype (middle) or IFNR1 blocking antibody (right). Symbols represent individual mice with bars indicating the mean of the group. In bar graphs, the data represent the average ± SD of three to six mice per group. All data are representative of two or more independent experiments. *P < 0.05.
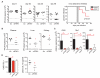
Fig. 3. IFNR1 blockade enhances control of persistent infection
WT mice were treated with isotype or IFNR1 blocking antibody beginning 1 day before LCMV-Cl13 infection. (A) Plasma virus titers at the indicated time points after infection. (B) Viral titers in plasma, liver, and kidney 30 days after infection. (C) Graphs indicate total numbers of IFN-γ–expressing and of multicytokine-producing (polyfunctional) LCMV-GP61–80–specific CD4 T cells and LCMV-GP33–41–specific CD8 T cells. (D) Plasma viral titers at day 30 after LCMV-Cl13 infection in mice that were either undepleted of cells and treated with isotype (iso/iso) or IFNR1 (αIFNR1/iso) blocking antibody or depleted of CD4 T cells (αCD4) or NK cells (αNK) before infection and IFNR1 blocking antibody treatment. (E) Plasma virus titers at the indicated time point after LCMV-Cl13 infection in mice that were either treated with isotype or IFNR1 blocking antibody with or without anti–IFN-γ (αIFN-γ). x axis labels indicate (top) IFNRI or isotype and (bottom) cell or IFN-γ depleting antibody treatments. Each symbol in the scatter plots represents an individual mouse with bars indicating the mean of the group. Dashed lines indicate the level of detection of the plaque assay (200 PFU). Data are representative of two or more independent experiments. *P < 0.05.
Fig. 4. Therapeutic IFNR1 antibody blockade dampens the immunosuppressive program and facilitates clearance of persistent infection
WT mice were treated with isotype or IFNR1 blocking antibody beginning on day 25 after LCMV-Cl13 infection. (A) Plasma viral titers at the indicated time points. The percent of mice at each time point exhibiting detectible virus titers is summarized in the kinetic graph to the right, with the shaded area representing the time of treatment. The data are combined from two experiments. (B) Viral titers in the plasma, liver, and kidney at day 46 after LCMV-Cl13 infection. (C) OAS, Mx1, and IRF7 mRNA expression relative to HPRT in splenocytes from naïve mice (white) or on day 30 after LCMV-Cl13 infection in mice treated with isotype (black) or IFNR1 (red) blocking antibody. (D) Graphs indicate GMFI of PD-L1 on splenic dendritic cells (left) and plasma IL-10 levels (right) on day 30 after LCMV-Cl13 infection in mice treated with isotype or IFNR1 blocking antibody. Each symbol in the scatter plot represents an individual mouse, and bar graphs indicate the average value ± SD. All data are representative of two to five independent experiments. *P < 0.05.
Comment in
- Immunology. An interferon paradox.
Odorizzi PM, Wherry EJ. Odorizzi PM, et al. Science. 2013 Apr 12;340(6129):155-6. doi: 10.1126/science.1237568. Science. 2013. PMID: 23580520 Free PMC article. - Infection: the interferon paradox.
Papatriantafyllou M. Papatriantafyllou M. Nat Rev Immunol. 2013 Jun;13(6):392. doi: 10.1038/nri3461. Epub 2013 May 7. Nat Rev Immunol. 2013. PMID: 23648970 No abstract available. - Interfering with type I interferon: a novel approach to purge persistent viral infection.
Wilson EB, Brooks DG. Wilson EB, et al. Cell Cycle. 2013 Sep 15;12(18):2919-20. doi: 10.4161/cc.26175. Epub 2013 Aug 23. Cell Cycle. 2013. PMID: 23974094 Free PMC article. No abstract available.
Similar articles
- Persistent LCMV infection is controlled by blockade of type I interferon signaling.
Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Teijaro JR, et al. Science. 2013 Apr 12;340(6129):207-11. doi: 10.1126/science.1235214. Science. 2013. PMID: 23580529 Free PMC article. - Interfering with type I interferon: a novel approach to purge persistent viral infection.
Wilson EB, Brooks DG. Wilson EB, et al. Cell Cycle. 2013 Sep 15;12(18):2919-20. doi: 10.4161/cc.26175. Epub 2013 Aug 23. Cell Cycle. 2013. PMID: 23974094 Free PMC article. No abstract available. - Negative regulation of type I IFN expression by OASL1 permits chronic viral infection and CD8⁺ T-cell exhaustion.
Lee MS, Park CH, Jeong YH, Kim YJ, Ha SJ. Lee MS, et al. PLoS Pathog. 2013;9(7):e1003478. doi: 10.1371/journal.ppat.1003478. Epub 2013 Jul 18. PLoS Pathog. 2013. PMID: 23874199 Free PMC article. - Confounding roles for type I interferons during bacterial and viral pathogenesis.
Carrero JA. Carrero JA. Int Immunol. 2013 Dec;25(12):663-9. doi: 10.1093/intimm/dxt050. Epub 2013 Oct 24. Int Immunol. 2013. PMID: 24158954 Free PMC article. Review. - Complexities of Type I Interferon Biology: Lessons from LCMV.
Suprunenko T, Hofer MJ. Suprunenko T, et al. Viruses. 2019 Feb 20;11(2):172. doi: 10.3390/v11020172. Viruses. 2019. PMID: 30791575 Free PMC article. Review.
Cited by
- Three modes of viral adaption by the heart.
Griffiths CD, Shah M, Shao W, Borgman CA, Janes KA. Griffiths CD, et al. Sci Adv. 2024 Nov 15;10(46):eadp6303. doi: 10.1126/sciadv.adp6303. Epub 2024 Nov 13. Sci Adv. 2024. PMID: 39536108 Free PMC article. - Circulating tumor cells: from new biological insights to clinical practice.
Gu X, Wei S, Lv X. Gu X, et al. Signal Transduct Target Ther. 2024 Sep 2;9(1):226. doi: 10.1038/s41392-024-01938-6. Signal Transduct Target Ther. 2024. PMID: 39218931 Free PMC article. Review. - Feasibility of Using a Type I IFN-Based Non-Animal Approach to Predict Vaccine Efficacy and Safety Profiles.
Abdel-Haq H. Abdel-Haq H. Vaccines (Basel). 2024 May 27;12(6):583. doi: 10.3390/vaccines12060583. Vaccines (Basel). 2024. PMID: 38932312 Free PMC article. Review. - Combined JAK inhibition and PD-1 immunotherapy for non-small cell lung cancer patients.
Mathew D, Marmarelis ME, Foley C, Bauml JM, Ye D, Ghinnagow R, Ngiow SF, Klapholz M, Jun S, Zhang Z, Zorc R, Davis CW, Diehn M, Giles JR, Huang AC, Hwang WT, Zhang NR, Schoenfeld AJ, Carpenter EL, Langer CJ, Wherry EJ, Minn AJ. Mathew D, et al. Science. 2024 Jun 21;384(6702):eadf1329. doi: 10.1126/science.adf1329. Epub 2024 Jun 21. Science. 2024. PMID: 38900877 Free PMC article. Clinical Trial. - JAK inhibition enhances checkpoint blockade immunotherapy in patients with Hodgkin lymphoma.
Zak J, Pratumchai I, Marro BS, Marquardt KL, Zavareh RB, Lairson LL, Oldstone MBA, Varner JA, Hegerova L, Cao Q, Farooq U, Kenkre VP, Bachanova V, Teijaro JR. Zak J, et al. Science. 2024 Jun 21;384(6702):eade8520. doi: 10.1126/science.ade8520. Epub 2024 Jun 21. Science. 2024. PMID: 38900864 Free PMC article. Clinical Trial.
References
- Barber DL, et al. Nature. 2006;439:682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI085043/AI/NIAID NIH HHS/United States
- 8UL1TR000077-04/TR/NCATS NIH HHS/United States
- R01 AI047868/AI/NIAID NIH HHS/United States
- R01 AI085043/AI/NIAID NIH HHS/United States
- HL108949/HL/NHLBI NIH HHS/United States
- R01 AI056154/AI/NIAID NIH HHS/United States
- P50 AR063020/AR/NIAMS NIH HHS/United States
- AI082975/AI/NIAID NIH HHS/United States
- R01 HL108949/HL/NHLBI NIH HHS/United States
- U01 AI082975/AI/NIAID NIH HHS/United States
- AI060567/AI/NIAID NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
- T32 AI060567/AI/NIAID NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials