Torsin mediates primary envelopment of large ribonucleoprotein granules at the nuclear envelope - PubMed (original) (raw)
Torsin mediates primary envelopment of large ribonucleoprotein granules at the nuclear envelope
Vahbiz Jokhi et al. Cell Rep. 2013.
Abstract
A previously unrecognized mechanism through which large ribonucleoprotein (megaRNP) granules exit the nucleus is by budding through the nuclear envelope (NE). This mechanism is akin to the nuclear egress of herpes-type viruses and is essential for proper synapse development. However, the molecular machinery required to remodel the NE during this process is unknown. Here, we identify Torsin, an AAA-ATPase that in humans is linked to dystonia, as a major mediator of primary megaRNP envelopment during NE budding. In torsin mutants, megaRNPs accumulate within the perinuclear space, and the messenger RNAs contained within fail to reach synaptic sites, preventing normal synaptic protein synthesis and thus proper synaptic bouton development. These studies begin to establish the cellular machinery underlying the exit of megaRNPs via budding, offer an explanation for the "nuclear blebbing" phenotype found in dystonia models, and provide an important link between Torsin and the synaptic phenotypes observed in dystonia.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
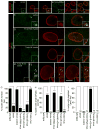
Figure 1. Morphology of nuclear DFz2C/Lam foci is disrupted in Torsin mutations
(Also see Fig. SF1). (A, B) Localization and morphology of DFz2C/Lam foci at the nuclei of S2 cells in (A) untreated cells, and (B) cells treated with Torsin-dsRNA. (C–F) Localization and morphology of nuclear DFz2C/LamC foci in larval muscles of (C) wild type and (D–F) larvae expressing (D) Torsin RNAi, (E) TorsinΔE, and (F) TorsinE→Q in muscles. F1 is a low magnification view. F2 shows a high magnification view of DFz2 puncta in the YZ and XY planes. (A–F) correspond to singe confocal slices. (G–I) Percentage of nuclear foci showing (G) normal organization of DFz2C/LamC, (H) the presence of small DFz2C puncta associated with the lamina (see text), and (I) the presence of thickenings of the lamina devoid of DFz2C signal. mus= muscle. N([number of nuclei;number of larvae])= [908;6],[731;6],[639;6],[802;6],[846;6],[733;6],[693;6]. Error bars represent ± SEM (***: p<0.0001). Calibration scale (μm)=A,B:14, (4μm for insets); C–F:10 (6μm for insets).
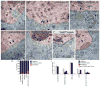
Figure 2. Ultrastructural organization of NE-associated megaRNPs is disrupted in Torsin mutations
(Also see Fig. SF2) (A–G) Electron micrographs of nuclear regions in (A–C) S2 cells, (D,F,G) larval body wall muscles, and (E) larval epithelial cells, showing NE-associated megaRNPs. Red=nucleus; blue=cytoplasm; green=perinuclear space. N=nucleus; C=cytoplasm. (A) Untreated S2 cell showing a normal nuclear focus (arrow) containing electron dense megaRNP granules. (B, C) NE of a Torsin-dsRNA-treated S2 cells, displaying megaRNPs tethered to the INM by collared necks (arrows), shown at (B) low and (C) high magnification. ONM=outer nuclear membrane, INM=inner nuclear membrane, ribo=ribosome. (D, E) NE in torsin null mutants also showing megaRNPs tethered to the INM (arrows). (F, G) NE in muscle cells expressing TorsinE→Q showing the presence of (F) a megaRNP (arrow) tethered to the INM, and (G) a large amorphous megaRNP (arrow) tightly apposed to the INM. mi=mitochondria. (H) Percentage of megaRNP granules present in INM invaginations (black), with collared necks (blue) and being large and amorphous (red). (I) Average number of megaRNP granules in INM invaginations (black), with collared necks (blue), being large and amorphous (red), per focus (white) N[number of granules;foci])= [159;36],[366;122],[207;33],[166;68],[181;88]. Error bars represent ±SEM. (*= p<0.05,**= p<0.001,***= p<0.0001). Calibration scale (μm)= A,B,D–G:0.5; and C:0.2.
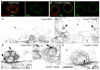
Figure 3. The TorsinE→Q protein accumulates at megaRNP collared necks
Also see Fig. SF1. (Also see Fig. SF3) (A, B) S2 cells expressing (A) wild type Torsin-Flag and (B) the TorsinE→Q-Flag, showing that Torsin-Flag accumulates at foci, and TorsinE→Q is punctate at the NE. (C–G) Electron micrographs of nuclear regions of S2 cells expressing (C, E) Torsin-SOG showing electron dense signal surrounding megaRNPs, or (D, F–G) TorsinE→Q-SOG showing that signal accumulates at (D, F) megaRNP collared necks or (G) at appositions of amorphous megaRNPs with the INM. Calibration scale (μm)= A,B:7; C,D:0.7; E–G:0.3.
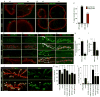
Figure 4. The distribution of mRNAs at the NE and synaptic sites in disrupted in torsin mutants
(Also see Fig. SF4). (A,B) FISH to body wall muscles showing the nuclear distribution of (A) par6 and (B) magi transcripts in wild type and torsin mutants. (C) Quantification of FISH signal outside the nucleus. N [nuclei;larvae]= [18;6],[18;6],[18;6],[14;6]. (D, E) FISH to body wall muscles showing the distribution of (D) par6 and (E) magi transcript at the NMJ in wild type and torsin null mutants. (F, G) Distribution of (F) Par6 and (G) Magi immunoreactivity at the NMJ in wild type and torsin mutants. (H, I) Quantification of postsynaptic (H) Par6 and (I) Magi immunoreactive signal, normalized to wild type control. N([NMJs;larvae])= [16;6],[18;6] for H and [17;6],[15;6] for I. (J) NMJs in wild type and torsin mutants labeled with anti-HRP and anti-DLG showing reduced size and increased ghost boutons (arrowheads) in torsin mutants. (K, L) Quantification of the number of (K) synaptic boutons and (L) ghost boutons. N([NMJs;larvae])= [19;10],[18;10],[19;10],[19;10],[20;10],[19;10],[19;10] for K,L. Error bars represent ±SEM (**= p<0.001, ***= p<0.0001). Calibration scale (μm)= A,B:3; D–G:10; J:20.
Similar articles
- Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling.
Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang YT, Li Q, Moore MJ, Budnik V. Speese SD, et al. Cell. 2012 May 11;149(4):832-46. doi: 10.1016/j.cell.2012.03.032. Cell. 2012. PMID: 22579286 Free PMC article. - The Role of Torsin AAA+ Proteins in Preserving Nuclear Envelope Integrity and Safeguarding Against Disease.
Rampello AJ, Prophet SM, Schlieker C. Rampello AJ, et al. Biomolecules. 2020 Mar 19;10(3):468. doi: 10.3390/biom10030468. Biomolecules. 2020. PMID: 32204310 Free PMC article. Review. - [Torsin 1A and the pathomechanism of torsion dystonia type 1].
Jurek M, Milewski M. Jurek M, et al. Postepy Biochem. 2015;61(1):35-41. Postepy Biochem. 2015. PMID: 26281352 Review. Polish. - Getting mRNA-Containing Ribonucleoprotein Granules Out of a Nuclear Back Door.
Parchure A, Munson M, Budnik V. Parchure A, et al. Neuron. 2017 Nov 1;96(3):604-615. doi: 10.1016/j.neuron.2017.10.020. Neuron. 2017. PMID: 29096075 Review. - Torsin ATPases: structural insights and functional perspectives.
Laudermilch E, Schlieker C. Laudermilch E, et al. Curr Opin Cell Biol. 2016 Jun;40:1-7. doi: 10.1016/j.ceb.2016.01.001. Epub 2016 Jan 21. Curr Opin Cell Biol. 2016. PMID: 26803745 Free PMC article. Review.
Cited by
- Differential Influence of Sample Sex and Neuronal Maturation on mRNA and Protein Transport in Induced Human Neurons.
Ding B, Akter M, Zhang CL. Ding B, et al. Front Mol Neurosci. 2020 Apr 3;13:46. doi: 10.3389/fnmol.2020.00046. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32317929 Free PMC article. - Mutant torsinA in the heterozygous DYT1 state compromises HSV propagation in infected neurons and fibroblasts.
György B, Cruz L, Yellen D, Aufiero M, Alland I, Zhang X, Ericsson M, Fraefel C, Li YC, Takeda S, Hyman BT, Breakefield XO. György B, et al. Sci Rep. 2018 Feb 2;8(1):2324. doi: 10.1038/s41598-018-19865-2. Sci Rep. 2018. PMID: 29396398 Free PMC article. - The ESCRT machinery: new roles at new holes.
Olmos Y, Carlton JG. Olmos Y, et al. Curr Opin Cell Biol. 2016 Feb;38:1-11. doi: 10.1016/j.ceb.2015.12.001. Epub 2016 Jan 15. Curr Opin Cell Biol. 2016. PMID: 26775243 Free PMC article. Review. - Structural Basis of Vesicle Formation at the Inner Nuclear Membrane.
Hagen C, Dent KC, Zeev-Ben-Mordehai T, Grange M, Bosse JB, Whittle C, Klupp BG, Siebert CA, Vasishtan D, Bäuerlein FJ, Cheleski J, Werner S, Guttmann P, Rehbein S, Henzler K, Demmerle J, Adler B, Koszinowski U, Schermelleh L, Schneider G, Enquist LW, Plitzko JM, Mettenleiter TC, Grünewald K. Hagen C, et al. Cell. 2015 Dec 17;163(7):1692-701. doi: 10.1016/j.cell.2015.11.029. Cell. 2015. PMID: 26687357 Free PMC article. - RNA biology of disease-associated microsatellite repeat expansions.
Rohilla KJ, Gagnon KT. Rohilla KJ, et al. Acta Neuropathol Commun. 2017 Aug 29;5(1):63. doi: 10.1186/s40478-017-0468-y. Acta Neuropathol Commun. 2017. PMID: 28851463 Free PMC article. Review.
References
- Barco A, Lopez de Armentia M, Alarcon JM. Synapse-specific stabilization of plasticity processes: the synaptic tagging and capture hypothesis revisited 10 years later. Neurosci Biobehav Rev. 2008;32:831–851. - PubMed
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous