Substituted methcathinones differ in transporter and receptor interactions - PubMed (original) (raw)
Comparative Study
Substituted methcathinones differ in transporter and receptor interactions
Amy J Eshleman et al. Biochem Pharmacol. 2013.
Abstract
The use of synthetic methcathinones, components of "bath salts," is a world-wide health concern. These compounds, structurally similar to methamphetamine (METH) and 3,4-methylendioxymethamphetamine (MDMA), cause tachycardia, hallucinations and psychosis. We hypothesized that these potentially neurotoxic and abused compounds display differences in their transporter and receptor interactions as compared to amphetamine counterparts. 3,4-Methylenedioxypyrovalerone and naphyrone had high affinity for radioligand binding sites on recombinant human dopamine (hDAT), serotonin (hSERT) and norepinephrine (hNET) transporters, potently inhibited [³H]neurotransmitter uptake, and, like cocaine, did not induce transporter-mediated release. Butylone was a lower affinity uptake inhibitor. In contrast, 4-fluoromethcathinone, mephedrone and methylone had higher inhibitory potency at uptake compared to binding and generally induced release of preloaded [³H]neurotransmitter from hDAT, hSERT and hNET (highest potency at hNET), and thus are transporter substrates, similar to METH and MDMA. At hNET, 4-fluoromethcathinone was a more efficacious releaser than METH. These substituted methcathinones had low uptake inhibitory potency and low efficacy at inducing release via human vesicular monoamine transporters (hVMAT2). These compounds were low potency (1) h5-HT(1A) receptor partial agonists, (2) h5-HT(2A) receptor antagonists, (3) weak h5-HT(2C) receptor antagonists. This is the first report on aspects of substituted methcathinone efficacies at serotonin (5-HT) receptors and in superfusion release assays. Additionally, the drugs had no affinity for dopamine receptors, and high-nanomolar to mid-micromolar affinity for hSigma1 receptors. Thus, direct interactions with hVMAT2 and serotonin, dopamine, and hSigma1 receptors may not explain psychoactive effects. The primary mechanisms of action may be as inhibitors or substrates of DAT, SERT and NET.
Published by Elsevier Inc.
Conflict of interest statement
The authors have no actual or potential conflicts of interest.
Figures
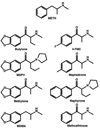
Figure 1
Structures of substituted methcathinones, MDMA, and METH.
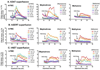
Figure 2
Time courses of [3H]neurotransmitter release induced by the substituted methcathinones 4-FMC, mephedrone and methylone from HEK-hDAT, -hSERT and -hNET cells. Data shown are representative experiments. Data are normalized to percent release of [3H]neurotransmitter remaining in cells at each time point. The last three buffer fractions prior to addition of drug and the 12 fractions in the presence of drug are shown.
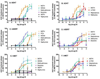
Figure 3
Substituted methcathinones, METH, and MDMA dose-response curves for release of [3H]neurotransmitter from HEK-hDAT (A,B), -hSERT (C,D) and -hNET (E,F) cells. The area under the curve (AUC) for each drug concentration was normalized to the maximal effect of METH for that experiment (hDAT and hNET) or the maximal effect of PCA (hSERT). A, C and E: Data show the lack of effect of minimal releasers compared to METH, methcathinone, MDMA and, for SERT, PCA. B, D, and F: Data show the effects of releasers at each or the transporters compared to METH.
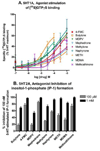
Figure 4
Effects of substituted methcathinones, METH and MDMA on h5-HT1A and h5-HT2A function. A. The h5-HT1A agonist assay, stimulation of [35S]GTPγS binding. The methcathinones were full to partial agonists, tested at concentrations ranging from 100 nM to 1 mM. B. The h5-HT2A antagonist assay, inhibition of 100 nM-5-HT-stimulated inositol-1-phosphate formation. The compounds had no agonist activity (data not shown). The substituted methcathinones were weak to full antagonists and were tested at concentrations ranging from 100 nM to 1 mM. The percent inhibition by each compound at 100 µM and 1 mM concentrations is shown.
Similar articles
- The profile of mephedrone on human monoamine transporters differs from 3,4-methylenedioxymethamphetamine primarily by lower potency at the vesicular monoamine transporter.
Pifl C, Reither H, Hornykiewicz O. Pifl C, et al. Eur J Pharmacol. 2015 May 15;755:119-26. doi: 10.1016/j.ejphar.2015.03.004. Epub 2015 Mar 11. Eur J Pharmacol. 2015. PMID: 25771452 - Structure-activity relationships of bath salt components: substituted cathinones and benzofurans at biogenic amine transporters.
Eshleman AJ, Nagarajan S, Wolfrum KM, Reed JF, Swanson TL, Nilsen A, Janowsky A. Eshleman AJ, et al. Psychopharmacology (Berl). 2019 Mar;236(3):939-952. doi: 10.1007/s00213-018-5059-5. Epub 2018 Nov 5. Psychopharmacology (Berl). 2019. PMID: 30397775 Free PMC article. - α-PPP and its derivatives are selective partial releasers at the human norepinephrine transporter: A pharmacological characterization of interactions between pyrrolidinopropiophenones and high and low affinity monoamine transporters.
Maier J, Rauter L, Rudin D, Niello M, Holy M, Schmid D, Wilson J, Blough BE, Gannon BM, Murnane KS, Sitte HH. Maier J, et al. Neuropharmacology. 2021 Jun 1;190:108570. doi: 10.1016/j.neuropharm.2021.108570. Epub 2021 Apr 20. Neuropharmacology. 2021. PMID: 33864800 - Neurotoxicology of Synthetic Cathinone Analogs.
Angoa-Pérez M, Anneken JH, Kuhn DM. Angoa-Pérez M, et al. Curr Top Behav Neurosci. 2017;32:209-230. doi: 10.1007/7854_2016_21. Curr Top Behav Neurosci. 2017. PMID: 27753008 Free PMC article. Review. - Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention.
Glennon RA. Glennon RA. Adv Pharmacol. 2014;69:581-620. doi: 10.1016/B978-0-12-420118-7.00015-9. Adv Pharmacol. 2014. PMID: 24484988 Free PMC article. Review.
Cited by
- 4-Isobutylmethcathinone-A Novel Synthetic Cathinone with High In Vitro Cytotoxicity and Strong Receptor Binding Preference of Enantiomers.
Paškan M, Rimpelová S, Svobodová Pavlíčková V, Spálovská D, Setnička V, Kuchař M, Kohout M. Paškan M, et al. Pharmaceuticals (Basel). 2022 Nov 30;15(12):1495. doi: 10.3390/ph15121495. Pharmaceuticals (Basel). 2022. PMID: 36558946 Free PMC article. - Central Effects of the Designer Drug Mephedrone in Mice-Basic Studies.
Serefko A, Bielecka-Papierz G, Talarek S, Szopa A, Skałecki P, Szewczyk B, Radziwoń-Zaleska M, Poleszak E. Serefko A, et al. Brain Sci. 2022 Jan 30;12(2):189. doi: 10.3390/brainsci12020189. Brain Sci. 2022. PMID: 35203952 Free PMC article. - Effects of dopaminergic and serotonergic compounds in rats trained to discriminate a high and a low training dose of the synthetic cathinone mephedrone.
Saber I, Milewski A, Reitz AB, Rawls SM, Walker EA. Saber I, et al. Psychopharmacology (Berl). 2019 Mar;236(3):1015-1029. doi: 10.1007/s00213-019-05241-z. Epub 2019 Apr 13. Psychopharmacology (Berl). 2019. PMID: 30980094 Free PMC article. - The effect of mephedrone on human neuroblastoma and astrocytoma cells.
Alanazi IM, Alzahrani AR, Alsaad MA, Moqeem AL, Hamdi AM, Taher MM, Watson DG, Helen Grant M. Alanazi IM, et al. Saudi Pharm J. 2024 Apr;32(4):102011. doi: 10.1016/j.jsps.2024.102011. Epub 2024 Feb 28. Saudi Pharm J. 2024. PMID: 38454918 Free PMC article. - Reinforcing and neurochemical effects of the "bath salts" constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats.
Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. Schindler CW, et al. Psychopharmacology (Berl). 2016 May;233(10):1981-90. doi: 10.1007/s00213-015-4057-0. Epub 2015 Aug 29. Psychopharmacology (Berl). 2016. PMID: 26319160 Free PMC article.
References
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011;49:499–505. - PubMed
- Striebel JM, Pierre JM. Acute psychotic sequelae of “bath salts”. Schizophr Res. 2011;133:259–260. - PubMed
- Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive “bath salts” intoxication with methylenedioxypyrovalerone. Am J Med. 2012;125:854–858. - PubMed
- Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. - PubMed
- Derungs A, Schietzel S, Meyer MR, Maurer HH, Krahenbuhl S, Liechti ME. Sympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone) Clin Toxicol (Phila) 2011;49:691–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources