Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease - PubMed (original) (raw)
Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease
Eric P Nagele et al. PLoS One. 2013.
Abstract
The presence of self-reactive IgG autoantibodies in human sera is largely thought to represent a breakdown in central tolerance and is typically regarded as a harbinger of autoimmune pathology. In the present study, immune-response profiling of human serum from 166 individuals via human protein microarrays demonstrates that IgG autoantibodies are abundant in all human serum, usually numbering in the thousands. These IgG autoantibodies bind to human antigens from organs and tissues all over the body and their serum diversity is strongly influenced by age, gender, and the presence of specific diseases. We also found that serum IgG autoantibody profiles are unique to an individual and remarkably stable over time. Similar profiles exist in rat and swine, suggesting conservation of this immunological feature among mammals. The number, diversity, and apparent evolutionary conservation of autoantibody profiles suggest that IgG autoantibodies have some important, as yet unrecognized, physiological function. We propose that IgG autoantibodies have evolved as an adaptive mechanism for debris-clearance, a function consistent with their apparent utility as diagnostic indicators of disease as already established for Alzheimer's and Parkinson's diseases.
Conflict of interest statement
Competing Interests: RGN is a stockholder in Durin Technologies, Inc., a small biotech company that is developing disease diagnostics using autoantibodies as biomarkers, and has filed patents on this technology. EPN is a paid consultant of Durin Technologies. This study was funded by the Foundation Venture Capital Group. There are no further patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures
Figure 1. Representative images of human protein microarrays.
(Left) Small portion of a protein microarray containing 9,480 native human proteins probed with blocking buffer and secondary antibody as a quality control. (Right) Protein microarray probed with an individual human serum sample under the same conditions. Manufacturer’s controls (white boxes), gradient of IgG positive controls (green boxes), anti-human IgG controls (blue boxes) and examples of immunopositive reactions (yellow boxes) are indicated. In this study, all arrays were scanned at PMT setting of 600. Under this condition, the relative fluorescence unit (RFU) at saturation is around 75000 as exemplified by IgG controls (green boxes). The RFUs of BSA negative controls are under 1000. The coefficient of variance between duplicate spots averaged 4.7% ∼ 8.8%.
Figure 2. Representative autoantibody data showing their relative abundance in healthy individuals.
An example of the reactions of four highly prevalent autoantibodies in a population of healthy subjects is presented. The X axis indicates individual samples arranged in order of increasing age from left to right. Relative fluorescence units (RFUs) (Y axis) reflect the resulting antigen-antibody reaction detected on the protein microarrays. Four specific autoantigens [tripartite motif-containing 21 (TRIM21), APEX nuclease (apurinic/apyrimidinic endonuclease) 2 (APEX2), General transcription factor II-I and SERPINE1 mRNA binding protein 1 (SERBP1)] were chosen due to their high prevalence in > 60% of the healthy population. The clear lamination of signal intensities shown by each autoantibody-antigen reaction suggests comparable levels of these autoantibodies in the blood across the population
Figure 3. Dot blot confirmation of serum IgG auto-reactivity.
Two identified antigens of serum autoantibodies, PTCD2 and ICAM4, were spotted onto nitrocellulose and probed with serum identical to that used on the protein microarrays. Serial dilution with diminishing immunoreactivity confirmed the presence and activity of IgG autoantibodies in human sera.
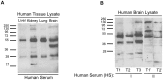
Figure 4. Serum IgG autoantibodies consistently bind to diverse antigens in many human organs. A.
Human liver, kidney, lung, and brain tissue lysates were separated by SDS-PAGE and then probed with human serum. As indicated by the many immunoreactive protein bands of differing molecular weights, serum IgG autoantibodies bind to a variety of common and organ-specific SDS-denatured antigens. B. In western blots, proteins from human brain extract were probed with human serum (HS-I and HS-II) as primary antibodies. Serum samples at three timepoints, T1, T2 and T3, were collected from the same individual (HS-I) over a period of four years. Serum samples T1’ and T2’ were collected from a different individual (HS-II) over a period of two years. Consistent patterns of serum IgG immunoreactivity were specific to individuals and appeared to remain stable over time.
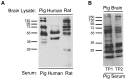
Figure 5. Serum IgG autoantibodies are detected in all mammals tested.
A. Pig, human, and rat brain lysates were separated by SDS-PAGE and then probed with their respective species-specific serum as a primary antibody. Each species possessed IgG autoantibodies that bound to a variety of SDS-denatured brain self-antigens. B. Proteins from pig brain extract were probed with serum collected from a single pig at two timepoints, TP1 and TP2, spanning three months. The majority of the pig auto-reactive IgG antibodies remain the same.
Similar articles
- Non-immunoglobulin serum proteins prevent the binding of IgG from normal rats and from rats with Th2-mediated autoimmune glomerulonephritis to various autoantigens including glomerular antigens.
Druet E, Praddaude F, Druet P, Dietrich G. Druet E, et al. Eur J Immunol. 1998 Jan;28(1):183-92. doi: 10.1002/(SICI)1521-4141(199801)28:01<183::AID-IMMU183>3.0.CO;2-O. Eur J Immunol. 1998. PMID: 9485198 - Natural autoantibodies are involved in the haemolytic anaemia of NZB mice.
Hentati B, Payelle-Brogard B, Jouanne C, Avrameas S, Ternynck T. Hentati B, et al. J Autoimmun. 1994 Aug;7(4):425-39. doi: 10.1006/jaut.1994.1031. J Autoimmun. 1994. PMID: 7980846 - Brain-reactive autoantibodies are nearly ubiquitous in human sera and may be linked to pathology in the context of blood-brain barrier breakdown.
Levin EC, Acharya NK, Han M, Zavareh SB, Sedeyn JC, Venkataraman V, Nagele RG. Levin EC, et al. Brain Res. 2010 Jul 23;1345:221-32. doi: 10.1016/j.brainres.2010.05.038. Epub 2010 Jun 11. Brain Res. 2010. PMID: 20546711 - Novel Concepts of Altered Immunoglobulin G Galactosylation in Autoimmune Diseases.
Dekkers G, Rispens T, Vidarsson G. Dekkers G, et al. Front Immunol. 2018 Mar 19;9:553. doi: 10.3389/fimmu.2018.00553. eCollection 2018. Front Immunol. 2018. PMID: 29616041 Free PMC article. Review. - Utility of autoantibodies as biomarkers for diagnosis and staging of neurodegenerative diseases.
DeMarshall C, Sarkar A, Nagele EP, Goldwaser E, Godsey G, Acharya NK, Nagele RG. DeMarshall C, et al. Int Rev Neurobiol. 2015;122:1-51. doi: 10.1016/bs.irn.2015.05.005. Epub 2015 Jun 19. Int Rev Neurobiol. 2015. PMID: 26358889 Review.
Cited by
- Antinuclear Autoantibodies in Health: Autoimmunity Is Not a Synonym of Autoimmune Disease.
Pashnina IA, Krivolapova IM, Fedotkina TV, Ryabkova VA, Chereshneva MV, Churilov LP, Chereshnev VA. Pashnina IA, et al. Antibodies (Basel). 2021 Feb 25;10(1):9. doi: 10.3390/antib10010009. Antibodies (Basel). 2021. PMID: 33668697 Free PMC article. Review. - Autoantibodies targeting neuronal proteins as biomarkers for neurodegenerative diseases.
Kocurova G, Ricny J, Ovsepian SV. Kocurova G, et al. Theranostics. 2022 Mar 28;12(7):3045-3056. doi: 10.7150/thno.72126. eCollection 2022. Theranostics. 2022. PMID: 35547759 Free PMC article. Review. - Natural antibodies - facts known and unknown.
Palma J, Tokarz-Deptuła B, Deptuła J, Deptuła W. Palma J, et al. Cent Eur J Immunol. 2018;43(4):466-475. doi: 10.5114/ceji.2018.81354. Epub 2018 Dec 31. Cent Eur J Immunol. 2018. PMID: 30799995 Free PMC article. Review. - Immune System Sex Differences May Bridge the Gap Between Sex and Gender in Fibromyalgia.
Meester I, Rivera-Silva GF, González-Salazar F. Meester I, et al. Front Neurosci. 2020 Jan 17;13:1414. doi: 10.3389/fnins.2019.01414. eCollection 2019. Front Neurosci. 2020. PMID: 32009888 Free PMC article. - Age-related Autoimmune Changes in Lacrimal Glands.
de Souza RG, de Paiva CS, Alves MR. de Souza RG, et al. Immune Netw. 2019 Feb 27;19(1):e3. doi: 10.4110/in.2019.19.e3. eCollection 2019 Feb. Immune Netw. 2019. PMID: 30838158 Free PMC article. Review.
References
- Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, et al. (1997) The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol 27: 1557–1563. - PubMed
- Hooijkaas H, Benner R, Pleasants JR, Wostmann BS (1984) Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered "antigen-free" diet. Eur J Immunol 14: 1127–1130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by funding from the Foundation Venture Capital Group “ a New Jersey Health Foundation Affiliate. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous