Relationship of DNA degradation by Saccharomyces cerevisiae exonuclease 1 and its stimulation by RPA and Mre11-Rad50-Xrs2 to DNA end resection - PubMed (original) (raw)
Relationship of DNA degradation by Saccharomyces cerevisiae exonuclease 1 and its stimulation by RPA and Mre11-Rad50-Xrs2 to DNA end resection
Elda Cannavo et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Homologous recombination is a major pathway for repair of DNA double-strand breaks. This repair process is initiated by resection of the 5′-terminated strand at the break site. In yeast, resection is carried out by three nucleolytic complexes: Mre11-Rad50-Xrs2, which functions at the initial step and also stimulates the two processive pathways, Sgs1-Dna2 and Exonuclease 1 (Exo1). Here we investigated the relationship between the three resection pathways with a focus on Exo1. Exo1 preferentially degrades the 5′-terminal stand of duplex DNA that is single stranded at the 3′ end, in agreement with its role downstream of the Mre11-Rad50-Xrs2 complex. Replication protein A (RPA) stimulates DNA end resection by Exo1 by both preventing nonspecific binding of Exo1 to and preventing degradation of single-stranded DNA. Nucleolytic degradation of DNA by Exo1 is inhibited by the helicase-deficient Sgs1 K706A mutant protein and, reciprocally, the nuclease-deficient Exo1 D173A mutant protein inhibits DNA unwinding by Sgs1. Thus, the activities of Sgs1 and Exo1 at DNA ends are mutually exclusive, establishing biochemically that both machineries function independently in DNA end processing. We also reconstituted Sgs1-Top3-Rmi1-RPA-Dna2 and Exo1 resection reactions both individually and combined, either with or without the Mre11-Rad50-Xrs2 complex. We show that the yeast Sgs1-Dna2 and Exo1 pathways do not stimulate one another and function as independent and separate DNA end-processing machineries, even in the presence of the stimulatory Mre11-Rad50-Xrs2 complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
RPA blocks degradation of ssDNA by Exo1. (A) FLAG-tagged wild-type Exo1 (210 ng) and FLAG-tagged nuclease-dead Exo1 (D173A, 140 ng) used in this study. (B) ssDNA and dsDNA (1 nM each), 32P labeled at the 5′ end, were used to analyze the nuclease activity of Exo1 (0.1, 0.2, 0.4, 0.8, 1.5, and 3 nM, respectively) in the absence or presence of RPA (22.5 nM). (C) Quantification of experiments as shown in B. Error bars show SE.
Fig. 2.
In the presence of RPA, resection of linear plasmid-length dsDNA by Exo1 produces kilobase-sized ssDNA. (A) Nuclease activity of Exo1 (2.7 nM) as a function of time (0.5, 1, 2, 4, 6, 10, 15, 20, and 30 min). pUC19 dsDNA (blunt, 1 nM), 32P labeled at the 3′ end, was the substrate. “Heat” refers to ssDNA produced by heat denaturation of the pUC19 dsDNA. (B) Reaction as in A carried out in the presence of RPA (0.4 μM). (C) Illustration summarizing results from A and B, showing the intermediates and products of dsDNA degradation by Exo1 in the presence or absence of RPA.
Fig. 3.
Exo1 preferentially degrades dsDNA resected at the 5′ end to produce an ssDNA tail at the 3′ end. (A) Quantification of Exo1 nuclease activity, in the presence of RPA (3 μM), using unlabeled pUC19 dsDNA (7.6 nM) that either was blunt or contained an ssDNA overhang (4 nt) at either 3′ or 5′ ends. Error bars indicate SE. (B) A representative experiment showing degradation of pUC19 dsDNA with a 5′-ssDNA overhang of 3 nt (7 nM, 32P labeled at the 3′ end) by Exo1 (0.05, 0.15, 0.45, 1.3, 4, and 12 nM, respectively) in the presence of RPA (2.8 μM). “D173A”: The nuclease-deficient Exo1 D173A mutant was used instead of wild-type Exo1 (12 nM). “Heat”: Heat-denatured substrate. (C) Illustration summarizing results from B showing degradation by Exo1 of a dsDNA substrate with 5′-end ssDNA overhangs. (D) Quantification of Exo1 nuclease activity on 3.0 kb unlabeled dsDNA (7.6 nM) containing an ssDNA overhang of 4 nt at both 5′ ends (squares) vs. dsDNA with a 4-nt 5′ overhang at one end and a 3′ (circles) or 5′ (triangles) overhang of ∼100 nt at the other end. The reactions were carried out in the presence of RPA (3 μM). Error bars show SE.
Fig. 4.
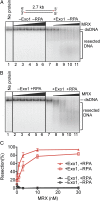
Mre11-Rad50-Xrs2 complex stimulates resection of dsDNA by Exo1. (A) A representative experiment with blunt-ended pUC19 dsDNA (1 nM, 32P labeled at the 3′ end) showing degradation by Exo1 (0.4 nM, where indicated) and its stimulation by MRX [1, 3, 10, 30, and 100 nM (lanes 2–6) and 1, 3, 10, and 30 nM (lanes 8–11), respectively]. (B) Reaction as in A carried out in the presence of RPA (0.4 μM). (C) Quantitation of experiments as in A and B. Error bars show SE.
Fig. 5.
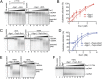
Sgs1 does not stimulate resection of dsDNA by Exo1. (A) Nuclease assays with Exo1 (0.35, 0.53, 0.8, 1.2, and 1.8 nM), RPA (0.4 μM), and either without (lanes 2–6) or with Sgs1 (0.1 nM, lanes 8–13) in low-salt buffer. Blunt-ended pUC19 dsDNA (1 nM), 32P labeled at the 3′ end, was used. (B) Quantification of experiments as shown in A. Error bars show SE. (C) Nuclease assays with Exo1 (0.53, 0.8, 1.2, 1.8, and 2.7 nM), RPA (0.4 μM), and either without (lanes 2–6) or with Sgs1 (0.5 nM) and Top3-Rmi1 (5 nM, lanes 9–14, respectively), in standard buffer. Substrate is as in A. (D) Quantification of experiments as shown in C. Error bars show SE. (E) Nuclease assay carried out with Exo1 (0.5, 1, 2, 3, and 4 nM), RPA (0.4 μM), and either without (lanes 2–6) or with helicase-dead Sgs1 K706A (20 nM, lanes 8–12). Substrate is as in A. (F) Increasing amounts of nuclease-dead Exo1 D173A (0.53, 0.8, 1.2, 1.8, 2.7, 4, and 8 nM) were added to reactions containing Sgs1 (0.5 nM) and/or Top3-Rmi1 (5 nM), as indicated, in the presence of RPA (0.4 μM). Substrate is as in A.
Fig. 6.
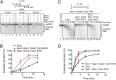
Exo1 does not stimulate DNA end resection by Dna2, Sgs1, and Top3-Rmi1. (A) Nuclease assays with Exo1 (7 nM), Dna2 (7 nM), Sgs1 (7 nM), Top3-Rmi1 (14 nM), and unlabeled pUC19 dsDNA containing 4-nt ssDNA overhangs at the 5′ ends (3.8 nM) in the presence of RPA (3 μM) for 4, 8, or 12 min. The gel is an inverted image of ethidium bromide-stained DNA. (B) Quantification of experiments as shown in A. Error bars show SE. (C) Nuclease assays with Exo1 (1 nM), Sgs1 (1 nM), Top3-Rmi1 (3 nM), Dna2 (1 nM), Mre11-Rad50-Xrs2 (25 nM), as indicated, for 1, 2, 4, 8, and 16 min in the presence of RPA (0.4 μM). Blunt-ended pUC19 dsDNA (1 nM), 32P labeled at the 3′ end, was used. (D) Quantification of experiments as shown in C. Error bars show SE.
Similar articles
- A DNA nick at Ku-blocked double-strand break ends serves as an entry site for exonuclease 1 (Exo1) or Sgs1-Dna2 in long-range DNA end resection.
Wang W, Daley JM, Kwon Y, Xue X, Krasner DS, Miller AS, Nguyen KA, Williamson EA, Shim EY, Lee SE, Hromas R, Sung P. Wang W, et al. J Biol Chem. 2018 Nov 2;293(44):17061-17069. doi: 10.1074/jbc.RA118.004769. Epub 2018 Sep 17. J Biol Chem. 2018. PMID: 30224356 Free PMC article. - DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2.
Cejka P, Cannavo E, Polaczek P, Masuda-Sasa T, Pokharel S, Campbell JL, Kowalczykowski SC. Cejka P, et al. Nature. 2010 Sep 2;467(7311):112-6. doi: 10.1038/nature09355. Nature. 2010. PMID: 20811461 Free PMC article. - Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks.
Shim EY, Chung WH, Nicolette ML, Zhang Y, Davis M, Zhu Z, Paull TT, Ira G, Lee SE. Shim EY, et al. EMBO J. 2010 Oct 6;29(19):3370-80. doi: 10.1038/emboj.2010.219. Epub 2010 Sep 10. EMBO J. 2010. PMID: 20834227 Free PMC article. - DNA End Resection: Nucleases Team Up with the Right Partners to Initiate Homologous Recombination.
Cejka P. Cejka P. J Biol Chem. 2015 Sep 18;290(38):22931-8. doi: 10.1074/jbc.R115.675942. Epub 2015 Jul 31. J Biol Chem. 2015. PMID: 26231213 Free PMC article. Review. - Structure-function relationships of the Mre11 protein in the control of DNA end bridging and processing.
Marsella A, Cassani C, Casari E, Tisi R, Longhese MP. Marsella A, et al. Curr Genet. 2019 Feb;65(1):11-16. doi: 10.1007/s00294-018-0861-5. Epub 2018 Jun 19. Curr Genet. 2019. PMID: 29922906 Review.
Cited by
- Cdc24 Is Essential for Long-range End Resection in the Repair of Double-stranded DNA Breaks.
Zhang H, Hua Y, Li R, Kong D. Zhang H, et al. J Biol Chem. 2016 Nov 25;291(48):24961-24973. doi: 10.1074/jbc.M116.755991. Epub 2016 Oct 11. J Biol Chem. 2016. PMID: 27729451 Free PMC article. - CaMKK2 and CHK1 phosphorylate human STN1 in response to replication stress to protect stalled forks from aberrant resection.
Jaiswal RK, Lei KH, Chastain M, Wang Y, Shiva O, Li S, You Z, Chi P, Chai W. Jaiswal RK, et al. Nat Commun. 2023 Nov 30;14(1):7882. doi: 10.1038/s41467-023-43685-2. Nat Commun. 2023. PMID: 38036565 Free PMC article. - Recombinational Repair of Nuclease-Generated Mitotic Double-Strand Breaks with Different End Structures in Yeast.
Gamble D, Shaltz S, Jinks-Robertson S. Gamble D, et al. G3 (Bethesda). 2020 Oct 5;10(10):3821-3829. doi: 10.1534/g3.120.401603. G3 (Bethesda). 2020. PMID: 32826304 Free PMC article. - A DNA nick at Ku-blocked double-strand break ends serves as an entry site for exonuclease 1 (Exo1) or Sgs1-Dna2 in long-range DNA end resection.
Wang W, Daley JM, Kwon Y, Xue X, Krasner DS, Miller AS, Nguyen KA, Williamson EA, Shim EY, Lee SE, Hromas R, Sung P. Wang W, et al. J Biol Chem. 2018 Nov 2;293(44):17061-17069. doi: 10.1074/jbc.RA118.004769. Epub 2018 Sep 17. J Biol Chem. 2018. PMID: 30224356 Free PMC article. - CtIP promotes the motor activity of DNA2 to accelerate long-range DNA end resection.
Ceppi I, Howard SM, Kasaciunaite K, Pinto C, Anand R, Seidel R, Cejka P. Ceppi I, et al. Proc Natl Acad Sci U S A. 2020 Apr 21;117(16):8859-8869. doi: 10.1073/pnas.2001165117. Epub 2020 Apr 2. Proc Natl Acad Sci U S A. 2020. PMID: 32241893 Free PMC article.
References
- Kramer KM, Haber JE. New telomeres in yeast are initiated with a highly selected subset of TG1-3 repeats. Genes Dev. 1993;7(12A):2345–2356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous