Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice - PubMed (original) (raw)
Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice
Shigeki Moriguchi et al. PLoS One. 2013.
Erratum in
- PLoS One. 2014;9(1). doi:10.1371/annotation/9fdc3705-7112-4382-a3a2-dcde33229272
Expression of concern in
- Expression of Concern: Stimulation of the Sigma-1 Receptor by DHEA Enhances Synaptic Efficacy and Neurogenesis in the Hippocampal Dentate Gyrus of Olfactory Bulbectomized Mice.
PLOS ONE Editors. PLOS ONE Editors. PLoS One. 2023 Aug 15;18(8):e0290363. doi: 10.1371/journal.pone.0290363. eCollection 2023. PLoS One. 2023. PMID: 37582118 Free PMC article. No abstract available.
Abstract
Dehydroepiandrosterone (DHEA) is the most abundant neurosteroid synthesized de novo in the central nervous system. We previously reported that stimulation of the sigma-1 receptor by DHEA improves cognitive function by activating calcium/calmodulin-dependent protein kinase II (CaMKII), protein kinase C and extracellular signal-regulated kinase in the hippocampus in olfactory bulbectomized (OBX) mice. Here, we asked whether DHEA enhances neurogenesis in the subgranular zone of the hippocampal dentate gyrus (DG) and improves depressive-like behaviors observed in OBX mice. Chronic treatment with DHEA at 30 or 60 mg/kg p.o. for 14 days significantly improved hippocampal LTP impaired in OBX mice concomitant with increased CaMKII autophosphorylation and GluR1 (Ser-831) phosphorylation in the DG. Chronic DHEA treatment also ameliorated depressive-like behaviors in OBX mice, as assessed by tail suspension and forced swim tests, while a single DHEA treatment had no affect. DHEA treatment also significantly increased the number of BrdU-positive neurons in the subgranular zone of the DG of OBX mice, an increase inhibited by treatment with NE-100, a sigma-1 receptor antagonist. DHEA treatment also significantly increased phosphorylation of Akt (Ser-473), Akt (Ser-308) and ERK in the DG. Furthermore, GSK-3β (Ser-9) phosphorylation increased in the DG of OBX mice possibly accounting for increased neurogenesis through Akt activation. Finally, we confirmed that DHEA treatment of OBX mice increases the number of BrdU-positive neurons co-expressing β-catenin, a downstream GSK-3βtarget. Overall, we conclude that sigma-1 receptor stimulation by DHEA ameliorates OBX-induced depressive-like behaviors by increasing neurogenesis in the DG through activation of the Akt/GSK-3β/β-catenin pathway.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. DHEA ameliorates perturbed LTP in the DG of OBX mice.
A: Representative fEPSPs were recorded from the DG in sham-operated mice, OBX mice, DHEA (60 mg/kg p.o.)-treated OBX mice, and DHEA plus NE-100 (1 mg/kg p.o.)-treated OBX mice. B: Changes in fEPSP slope following HFS recorded in the DG were attenuated in OBX compared with sham-operated mice. DHEA treatment significantly improved LTP in OBX mice. NE-100 significantly inhibited enhancement of LTP mediated by DHEA in OBX mice. C: Changes in fEPSP slope following HFS are shown in sham-operated, OBX, DHEA-treated OBX, and DHEA plus NE-100-treated OBX mice at 1 or 60 min. Vertical lines show SEM. **, p<0.01 versus sham-operated mice. ++, p<0.01 versus OBX mice. ##, p<0.01 versus DHEA-treated OBX mice.
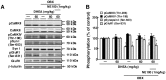
Figure 2. DHEA rescues reduced autophosphorylation of CaMKIIα (Thr-286) and phosphorylation of GluR1 (Ser-831) but does not alter phosphorylation of CaMKIV (Thr-196) or synapsin I (Ser-603) in the DG of OBX mice.
A: Representative images of immunoblots using antibodies against autophosphorylated CaMKII, CaMKII, phosphorylated CaMKIV (Thr-196), phosphorylated synapsin I (Ser-606), synapsin I, phosphorylated GluR1 (Ser-831) and GluR1. B: Quantitative analyses of autophosphorylated CaMKIIα (Thr-286) and phosphorylated CaMKIV (Thr-196), synapsin I (Ser-606) and GluR1 (Ser-831). Vertical lines show SEM. **, p<0.01 versus sham-operated mice. ++, p<0.01 versus OBX mice. ##, p<0.01 versus DHEA-treated OBX mice.
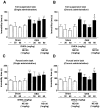
Figure 3. DHEA antagonizes depressive-like behaviors in OBX mice.
A: Immobility time in a tail suspension test was measured following a single administration of DHEA at 30 or 60 mg/kg p.o. 14 days after OBX surgery. No difference in immobility time was observed among OBX, or DHEA-treated OBX mice (Mann-Whitney U-test, n = 5 per group). B: Immobility time in the tail suspension test was measured after repeated, chronic administration of DHEA at 30 or 60 mg/kg p.o. for 12–13 days, starting 14 days after OBX surgery. DHEA rescued immobility time in OBX mice. NE-100 (1 mg/kg p.o.) pre-administration significantly inhibited decreased immobility time seen following DHEA treatment of OBX mice (Mann-Whitney U-test, n = 5 per group). C: Immobility time in a forced swim test was measured following a single administration of DHEA at 30 or 60 mg/kg p.o. 14 days after OBX surgery. No difference in immobility time was observed between OBX, or DHEA-treated OBX (Mann-Whitney U-test, n = 5 per group). D: Immobility time in a forced swim test was measured after repeated DHEA administration at 30 or 60 mg/kg p.o. for 12–13 days, starting 14 days after OBX operation. DHEA rescued immobility time seen in OBX mice. Pre-administration of NE-100 (1 mg/kg p.o.) significantly inhibited DHEA-mediated rescue of immobility time in OBX mice (Mann-Whitney U-test, n = 5 per group). Vertical lines show SEM. *, p<0.05; **, p<0.01 versus sham-operated mice. +, p<0.05; ++, p<0.01 versus OBX mice. #, p<0.05 versus DHEA-treated OBX mice.
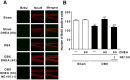
Figure 4. DHEA treatment enhances hippocampal neurogenesis in OBX mice.
A: Confocal microscopy images showing double staining for BrdU (green), NeuN (red) and merged images in hippocampal slices 30 days after OBX surgery (n = 8). B: Quantitative analyses of the number of BrdU/NeuN double-positive cells in the DG (n = 8). Vertical lines show SEM. **, p<0.01 versus sham-operated mice. ++, p<0.01 versus OBX mice. ##, p<0.01 versus DHEA-treated OBX mice.
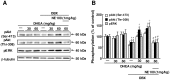
Figure 5. DHEA treatment restores reduced phosphorylation of Akt (Ser-473), Akt (Thr-308) and ERK in the DG of OBX mice.
A: Representative images of immunoblots using antibodies against phosphorylated Akt (Ser-473), phosphorylated Akt (Thr-308) and phosphorylated ERK. B: Quantitative analyses of phosphorylated Akt (Ser-473), phosphorylated Akt (Thr-308) and phosphorylated ERK. Vertical lines show SEM. **, p<0.01 versus sham-operated mice. +, p<0.05; ++, p<0.01versus OBX mice. ##, p<0.01 versus DHEA-treated OBX mice.
Figure 6. DHEA treatment antagonizes increased GSK-3β (Ser-9) phosphorylation seen in the DG of OBX mice and increases the number of BrdU-positive neurons showing nuclear β-catenin.
A: Representative images of immunoblots using antibodies against phosphorylated GSK-3β (Ser-9), phosphorylated mTOR (Ser-2448), phosphorylated CREB (Ser-133) and quantitative analyses of phosphorylated GSK-3β (Ser-9), phosphorylated mTOR (Ser-2448), and phosphorylated CREB (Ser-133). B: Confocal microscopy images showing double staining for BrdU (green), β-catenin (red) and merged images taken from hippocampal slices 30 days after OBX surgery. C: Quantitative analyses of the number of BrdU/β-catenin double-positive cells in the DG. Vertical lines show SEM. **, p<0.01 versus sham-operated mice. ++, p<0.01 versus OBX mice. ##, p<0.01 versus DHEA-treated OBX mice.
Similar articles
- Memantine Improves Depressive-like Behaviors via Kir6.1 Channel Inhibition in Olfactory Bulbectomized Mice.
Moriguchi S, Inagaki R, Shimojo H, Sugimura Y, Fukunaga K. Moriguchi S, et al. Neuroscience. 2020 Aug 21;442:264-273. doi: 10.1016/j.neuroscience.2020.06.002. Epub 2020 Jun 10. Neuroscience. 2020. PMID: 32531473 - Novel nootropic drug sunifiram improves cognitive deficits via CaM kinase II and protein kinase C activation in olfactory bulbectomized mice.
Moriguchi S, Tanaka T, Tagashira H, Narahashi T, Fukunaga K. Moriguchi S, et al. Behav Brain Res. 2013 Apr 1;242:150-7. doi: 10.1016/j.bbr.2012.12.054. Epub 2013 Jan 4. Behav Brain Res. 2013. PMID: 23295391 - [Neurochemical mechanisms of a novel Alzheimer's disease therapeutics on improvement of cognition and depressive behavior].
Shioda N, Yamamoto Y, Han F, Moriguchi S, Fukunaga K. Shioda N, et al. Yakugaku Zasshi. 2011 Apr;131(4):505-11. doi: 10.1248/yakushi.131.505. Yakugaku Zasshi. 2011. PMID: 21467789 Review. Japanese. - Stimulation of the Sigma-1 Receptor and the Effects on Neurogenesis and Depressive Behaviors in Mice.
Fukunaga K, Moriguchi S. Fukunaga K, et al. Adv Exp Med Biol. 2017;964:201-211. doi: 10.1007/978-3-319-50174-1_14. Adv Exp Med Biol. 2017. PMID: 28315273 Review.
Cited by
- CCNP Innovations in Neuropsychopharmacology Award: The psychopharmacology of psychedelics: where the brain meets spirituality.
Gobbi G. Gobbi G. J Psychiatry Neurosci. 2024 Sep 19;49(5):E301-E318. doi: 10.1503/jpn.240037. Print 2024 Sep-Oct. J Psychiatry Neurosci. 2024. PMID: 39299781 Free PMC article. Review. - Fenfluramine increases survival and reduces markers of neurodegeneration in a mouse model of Dravet syndrome.
Cha J, Filatov G, Smith SJ, Gammaitoni AR, Lothe A, Reeder T. Cha J, et al. Epilepsia Open. 2024 Feb;9(1):300-313. doi: 10.1002/epi4.12873. Epub 2023 Dec 22. Epilepsia Open. 2024. PMID: 38018342 Free PMC article. - miR-377-3p Regulates Hippocampal Neurogenesis via the Zfp462-Pbx1 Pathway and Mediates Anxiety-Like Behaviors in Prenatal Hypoxic Offspring.
Wang B, Zhu Y, Wei B, Zeng H, Zhang P, Li L, Wang H, Wu X, Zheng Y, Sun M. Wang B, et al. Mol Neurobiol. 2024 Apr;61(4):1920-1935. doi: 10.1007/s12035-023-03683-3. Epub 2023 Oct 11. Mol Neurobiol. 2024. PMID: 37817032 - Expression of Concern: Stimulation of the Sigma-1 Receptor by DHEA Enhances Synaptic Efficacy and Neurogenesis in the Hippocampal Dentate Gyrus of Olfactory Bulbectomized Mice.
PLOS ONE Editors. PLOS ONE Editors. PLoS One. 2023 Aug 15;18(8):e0290363. doi: 10.1371/journal.pone.0290363. eCollection 2023. PLoS One. 2023. PMID: 37582118 Free PMC article. No abstract available. - α7 Nicotinic acetylcholine receptor: a key receptor in the cholinergic anti-inflammatory pathway exerting an antidepressant effect.
Liu H, Zhang X, Shi P, Yuan J, Jia Q, Pi C, Chen T, Xiong L, Chen J, Tang J, Yue R, Liu Z, Shen H, Zuo Y, Wei Y, Zhao L. Liu H, et al. J Neuroinflammation. 2023 Mar 27;20(1):84. doi: 10.1186/s12974-023-02768-z. J Neuroinflammation. 2023. PMID: 36973813 Free PMC article. Review.
References
- Corwin J, Serby M, Conrad P, Rotrosen J (1985) Olfactory recognition deficit in Alzheimer’s disease and Parkinsonian dementia. IRCS Med Sci 13: 260.
- Koss E (1986) Olfactory dysfunction in Alzheimer’s disease. Dev Neuropsychol 2: 89–99.
- Warner MD, Peabody CA, Flattery JJ, Tinklenberg JR (1986) Olfactory deficits and Alzheimer’s disease. Biol Psychiatry 2: 116–118. - PubMed
- Kovacs T, Cairns NJ, Lantos PL (2001) Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stage. Neuroreport 12: 285–288. - PubMed
- Han F, Shioda N, Moriguchi S, Qin ZH, Fukunaga K (2008) The vanadium (LV) compound rescues septo-hippocampal cholinergic neurons from neurodegeneration in olfactory bulbectomized mice. Neuroscience 151: 671–679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health and Welfare of Japan (22390109 to K.F.; 20790398 to S.M.) and the Pharmacological Research Foundation, Tokyo (to S.M.), the Research Foundation for Pharmaceutical Sciences (to S.M.), the Smoking Research Foundation (to K.F.), the Takeda Science Foundation (to S.M.), the NISHINOMIYA Basic Research Fund (Japan) (to S.M.), the Suzuken Memorial Foundation (to S.M.), the HIROMI Medical Research Foundation (to S.M.). Funding was provided by the Tohoku University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous