Mitochondrial DNA replication proceeds via a 'bootlace' mechanism involving the incorporation of processed transcripts - PubMed (original) (raw)
Mitochondrial DNA replication proceeds via a 'bootlace' mechanism involving the incorporation of processed transcripts
Aurelio Reyes et al. Nucleic Acids Res. 2013 Jun.
Abstract
The observation that long tracts of RNA are associated with replicating molecules of mitochondrial DNA (mtDNA) suggests that the mitochondrial genome of mammals is copied by an unorthodox mechanism. Here we show that these RNA-containing species are present in living cells and tissue, based on interstrand cross-linking. Using DNA synthesis in organello, we demonstrate that isolated mitochondria incorporate radiolabeled RNA precursors, as well as DNA precursors, into replicating DNA molecules. RNA-containing replication intermediates are chased into mature mtDNA, to which they are thus in precursor-product relationship. While a DNA chain terminator rapidly blocks the labeling of mitochondrial replication intermediates, an RNA chain terminator does not. Furthermore, processed L-strand transcripts can be recovered from gel-extracted mtDNA replication intermediates. Therefore, instead of concurrent DNA and RNA synthesis, respectively, on the leading and lagging strands, preformed processed RNA is incorporated as a provisional lagging strand during mtDNA replication. These findings indicate that RITOLS is a physiological mechanism of mtDNA replication, and that it involves a 'bootlace' mechanism, in which processed transcripts are successively hybridized to the lagging-strand template, as the replication fork advances.
Figures
Figure 1.
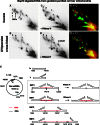
Psoralen cross-linking in vivo renders mtRIs resistant to RNase H. (A–D) DNA from cross-linked or control mitochondria were isolated, digested with BspHI and then, where indicated, digested further with RNase H, before separation by 2D-AGE. After transfer, the membrane was probed with probe r1 (see
Supplementary Data
for probe details). SMYs 1, 2 and 3 are SMY replication fork arcs, or their RNase H-modified counterparts. (E) schematic BspHI restriction map of rat mtDNA. To the right of the map are illustrated interpretations of species detected by probe r1, including typical intermediates of the three principle SMY arcs produced by BspHI digestion of rat mtDNA. In the case of SMY1 (inset), the effect of RNase H is interpreted according to whether the mtDNA was subjected to psoralen cross-linking in the organelle. Black lines represent DNA, red lines are RNA, red crosses indicate RNA/DNA psoralen-mediated interstrand cross-links, DNA/DNA cross-links are not shown for simplicity; a black cross is indicative of a blocked restriction site on one branch of the RI, owing to RNA/DNA hybrid. To the right of the gel images are merged false-colour images of A and C, and B and D, providing a direct comparison of the effect of RNase H on control and cross-linked samples: red—untreated (A and B), green—treated with RNase H (C and D). Species resistant to RNase H appear yellow; those modified or generated by RNase H appear red and green, respectively. BspHI was chosen because it produces a series of well-resolved SMY arcs covering almost the entire mitochondrial genome.
Figure 2.
The majority of in organello synthesized mtRIs have the mobility and RNase sensitivity of those present in vivo. After in organello labeling of rat liver mitochondria (6.6 nM [α32P]-dATP, 5 min, 1 mg protein per reaction), mtDNA was extracted, digested with BspHI and subjected to 2D-AGE. Phosphorimager analysis revealed a series of SMY replication fork arcs (A), substantially the same as those detected by Southern hybridization after probing with r1 (B). Note that probe r1 detects a specific fragment of mtDNA (i), whereas incubation of mitochondria with [α32P]-dATP labels all fragments of the mtDNA during the reaction (i, ii, iii). (C–E) In organello labeled rat liver mtDNA was digested with BlpI, separated by 2D-AGE, transferred to filter-membrane and phosphorimaged. Where indicated, samples were treated additionally, after restriction digestion, with RNase H or RNase T1. Interpretations of the arcs and spots appear immediately below the 2D gel images. SMY—SMY replication fork arc; b—origin-containing bubble arc of the RITOLS type [see (8), y—standard replication (y) fork arcs, e—eyebrow arc (8), mSMY—modified SMY, mb—modified bubble (22), me—modified eyebrow, 16 kb—late maturation intermediate where RNA remains at the restriction site at nucleotide 513, but not at 11 234, representative examples are illustrated. Also illustrated are the 1 n linear restriction fragments of 5.6 and 11 kb. Not illustrated are nicked (uncut) circles (nc).
Figure 3.
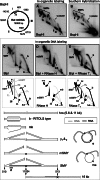
Pulse-chase experiments establish a precursor–product relationship between RNA-containing mtRIs and mature mtDNA. (A, B and D) Five-minute pulse-labeling of rat liver mitochondria with 6.6 nM [α32P]-dATP, (A, B) followed by chases of 10, 25, 40, 55, 115 or 175 min, with 660 µM unlabeled dATP or (D) following pre-incubation without [α32P]-dATP for 115 or 175 min. Extracted DNA was BlpI digested, separated by 1D (A) or 2D-AGE (B, D) and phosphorimaged after membrane transfer. Brackets demarcate the loss of signal on the replication bubble arc. (C) Quantitation of label in mtRIs, during chase, based on phosphorimaging. Values on vertical axes are based on signal from in organello labeling (nascent) normalized to Southern blot (steady state) signal for the different species analyzed. The resulting ratios for the chase samples were expressed as relative to no chase (0’ chase), set as 1.
Figure 4.
In organello labeling of mtRIs using an RNA precursor. (A–F) Isolated rat liver mitochondria were incubated for 10 min, 30 min, 1 h or 2 h with [α32P]-UTP. Extracted mtDNA was digested with BlpI, and treated with RNase H or RNase T1, as indicated, before 2D-AGE. (D*, E* and F*) Southern hybridizations, performed on duplicate samples incubated for 2 h prepared in parallel with the [α32P]-UTP labeling reactions. Longer exposures of panels A and B (the 10 and 30 min incubations) are shown to demonstrate that the gels were not blank.
Figure 5.
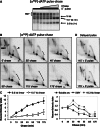
An RNA chain terminator rapidly inhibits nascent RNA formation in isolated mitochondria but affects mtDNA replication much more slowly. (A) 2D-AGE analysis of rat mtDNA labeled for 120 min with [α32P]-dUTP after pre-incubation without (control) or with 20 µM cordycepin triphosphate for 20 min. The intactness of the mtRIs is confirmed in
Supplementary Figure S5C
. (B) 2D-AGE analysis of rat mtDNA labeled for 5 min with [α32P]-dATP after pre-incubation without (control) or with 20 µM cordycepin triphosphate for 55 or 115 min. (C) Quantitation of the signals from the linear BlpI fragments of mtDNA (panel B and equivalent samples), during 3′ dATP treatment. Note the slow but gradual decrease in [α32P]-dATP incorporation into mtDNA. [α32P]-dATP incorporation was standardized to the level of steady-state mtRIs, based on Southern hybridization. (D) Steady-state levels, relative to zero time point, of mitochondrial rRNA (12S and 16S) and mRNA (COX2, ND1, ND3, ND5 and CYTB), after the indicated times of incubation with 20 µM cordycepin triphosphate. Measurements were based on Q-PCR values normalized to those for mtDNA.
Figure 6.
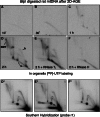
mtRIs are associated with processed transcripts. Gel-excised material representing the SMY arcs from the indicated portion of a 2D gel of _Bcl_I-digested mouse liver mtDNA, blot-hybridized to L-strand specific riboprobes: 1 (nt 14 903–15 339), 2, nt 13 874–15 339 or 3, nt 13 584–14 014 (panels 1–3, respectively). Black arrowheads indicate species co-migrating with previously characterized L-strand mRNAs. Gray arrowheads indicate the parental L-strand DNA from this restriction fragment (nt 12 034–16 180). Gel-excised material was left untreated (U), treated with RNase H (R) or DNase (D), and then separated by 1D-AGE following heat denaturation. A map of the relevant BclI fragment of mouse mtDNA is shown below the gel panels. NCR—major non-coding region; Cyt _b_—cytochrome b gene; ND5, ND6—NADH dehydrogenase 5, 6 genes. RNA5 comprises antisense tRNAGlu and ND6 conjoined with the sense strand of ND5, representing the mRNA for ND5. For a full description of these and other transcripts of mammalian mtDNA, see (24–27) and
Supplementary Figure S8
.
Figure 7.
The bootlace model of mtDNA Replication. Preformed (L-strand) transcripts, including complementary tRNA and mRNA hybridize to the template lagging strand of mammalian mtDNA as leading strand DNA synthesis proceeds. RNA recruitment is an ongoing process throughout the replication cycle, possibly mediated by specialized components of the mitochondrial (RITOLS) replisome. Incorporation of the transcripts 3′–5′ (A) avoids the formation of single-stranded (ss) sections of DNA, as indicated by the data reported here, whereas such segments would be unavoidable if the transcripts were laid down 5′–3′ (B).
Similar articles
- An overview of mammalian mitochondrial DNA replication mechanisms.
Yasukawa T, Kang D. Yasukawa T, et al. J Biochem. 2018 Sep 1;164(3):183-193. doi: 10.1093/jb/mvy058. J Biochem. 2018. PMID: 29931097 Free PMC article. Review. - Analysis of Replicating Mitochondrial DNA by In Organello Labeling and Two-Dimensional Agarose Gel Electrophoresis.
Holt IJ, Kazak L, Reyes A, Wood SR. Holt IJ, et al. Methods Mol Biol. 2016;1351:95-113. doi: 10.1007/978-1-4939-3040-1_8. Methods Mol Biol. 2016. PMID: 26530677 - Transcription mapping of the Ori L region reveals novel precursors of mature RNA species and antisense RNAs in rat mitochondrial genome.
Sbisà E, Tullo A, Nardelli M, Tanzariello F, Saccone C. Sbisà E, et al. FEBS Lett. 1992 Jan 27;296(3):311-6. doi: 10.1016/0014-5793(92)80311-4. FEBS Lett. 1992. PMID: 1371477 - Animal Mitochondrial DNA Replication.
Ciesielski GL, Oliveira MT, Kaguni LS. Ciesielski GL, et al. Enzymes. 2016;39:255-92. doi: 10.1016/bs.enz.2016.03.006. Epub 2016 May 9. Enzymes. 2016. PMID: 27241933 Free PMC article. Review. - Expression of catalytic mutants of the mtDNA helicase Twinkle and polymerase POLG causes distinct replication stalling phenotypes.
Wanrooij S, Goffart S, Pohjoismäki JL, Yasukawa T, Spelbrink JN. Wanrooij S, et al. Nucleic Acids Res. 2007;35(10):3238-51. doi: 10.1093/nar/gkm215. Epub 2007 Apr 22. Nucleic Acids Res. 2007. PMID: 17452351 Free PMC article.
Cited by
- Length heterogeneity at conserved sequence block 2 in human mitochondrial DNA acts as a rheostat for RNA polymerase POLRMT activity.
Tan BG, Wellesley FC, Savery NJ, Szczelkun MD. Tan BG, et al. Nucleic Acids Res. 2016 Sep 19;44(16):7817-29. doi: 10.1093/nar/gkw648. Epub 2016 Jul 19. Nucleic Acids Res. 2016. PMID: 27436287 Free PMC article. - Defects in mtDNA replication challenge nuclear genome stability through nucleotide depletion and provide a unifying mechanism for mouse progerias.
Hämäläinen RH, Landoni JC, Ahlqvist KJ, Goffart S, Ryytty S, Rahman MO, Brilhante V, Icay K, Hautaniemi S, Wang L, Laiho M, Suomalainen A. Hämäläinen RH, et al. Nat Metab. 2019 Oct;1(10):958-965. doi: 10.1038/s42255-019-0120-1. Epub 2019 Oct 7. Nat Metab. 2019. PMID: 32694840 - The mitochondrial R-loop.
Holt IJ. Holt IJ. Nucleic Acids Res. 2019 Jun 20;47(11):5480-5489. doi: 10.1093/nar/gkz277. Nucleic Acids Res. 2019. PMID: 31045202 Free PMC article. - Mitochondrial DNA maintenance in Drosophila melanogaster.
Rodrigues APC, Novaes AC, Ciesielski GL, Oliveira MT. Rodrigues APC, et al. Biosci Rep. 2022 Nov 30;42(11):BSR20211693. doi: 10.1042/BSR20211693. Biosci Rep. 2022. PMID: 36254835 Free PMC article. Review. - Circulating Mitochondrial DNA and Inter-Organelle Contact Sites in Aging and Associated Conditions.
Picca A, Guerra F, Calvani R, Romano R, Coelho-Junior HJ, Damiano FP, Bucci C, Marzetti E. Picca A, et al. Cells. 2022 Feb 15;11(4):675. doi: 10.3390/cells11040675. Cells. 2022. PMID: 35203322 Free PMC article. Review.
References
- Kornberg A, Baker TA. DNA Replication. 2nd edn. New York: W. H. Freeman & Co.; 1992.
- Clayton DA. Replication of animal mitochondrial DNA. Cell. 1982;28:693–705. - PubMed
- Kasamatsu H, Vinograd J. Unidirectionality of replication in mouse mitochondrial DNA. Nat. New Biol. 1973;241:103–105. - PubMed
- Crews S, Ojala D, Posakony J, Nishiguchi J, Attardi G. Nucleotide sequence of a region of human mitochondrial DNA containing the precisely identified origin of replication. Nature. 1979;277:192–198. - PubMed
- Tapper DP, Clayton DA. Mechanism of replication of human mitochondrial DNA. Localization of the 5′ ends of nascent daughter strands. J. Biol. Chem. 1981;256:5109–5115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources