Npas4 is activated by melatonin, and drives the clock gene Cry1 in the ovine pars tuberalis - PubMed (original) (raw)
Npas4 is activated by melatonin, and drives the clock gene Cry1 in the ovine pars tuberalis
A West et al. Mol Endocrinol. 2013 Jun.
Abstract
Seasonal mammals integrate changes in the duration of nocturnal melatonin secretion to drive annual physiologic cycles. Melatonin receptors within the proximal pituitary region, the pars tuberalis (PT), are essential in regulating seasonal neuroendocrine responses. In the ovine PT, melatonin is known to influence acute changes in transcriptional dynamics coupled to the onset (dusk) and offset (dawn) of melatonin secretion, leading to a potential interval-timing mechanism capable of decoding changes in day length (photoperiod). Melatonin offset at dawn is linked to cAMP accumulation, which directly induces transcription of the clock gene Per1. The rise of melatonin at dusk induces a separate and distinct cohort, including the clock-regulated genes Cry1 and Nampt, but little is known of the up-stream mechanisms involved. Here, we used next-generation sequencing of the ovine PT transcriptome at melatonin onset and identified Npas4 as a rapidly induced basic helix-loop-helix Per-Arnt-Sim domain transcription factor. In vivo we show nuclear localization of NPAS4 protein in presumptive melatonin target cells of the PT (α-glycoprotein hormone-expressing cells), whereas in situ hybridization studies identified acute and transient expression in the PT of Npas4 in response to melatonin. In vitro, NPAS4 forms functional dimers with basic helix loop helix-PAS domain cofactors aryl hydrocarbon receptor nuclear translocator (ARNT), ARNT2, and ARNTL, transactivating both Cry1 and Nampt ovine promoter reporters. Using a combination of 5'-deletions and site-directed mutagenesis, we show NPAS4-ARNT transactivation to be codependent upon two conserved central midline elements within the Cry1 promoter. Our data thus reveal NPAS4 as a candidate immediate early-response gene in the ovine PT, driving molecular responses to melatonin.
Figures
Figure 1.
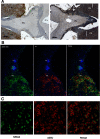
Npas4 mRNA Is quickly and Transiently Induced in Sheep after Melatonin Treatment and in SP Conditions. A, Quantitative ISH for Npas4 in the sheep PT housed in LPs (16 h light:8 h dark) after 1h30, 3h30, 6h30, and 9h30 melatonin (mel) or sham (Ct) treatment. Insert, representative autoradiographs after 1h30 treatment in melatonin and control group; scale bar, 200 μm. B, Quantitative ISH for Npas4 in the sheep PT every 4 hours in SPs (8 h light:16 h dark). Data show the mean relative optic density across 3 sections for each animal (n = 6 LP; n = 4 SP). Statistical differences between groups were analyzed after 2-way ANOVA followed by Bonferroni's post hoc test (***, P < .001).
Figure 2.
NPAS4 Is Detected in Nuclei of αGSU Expressing Ovine PT Cells. A, Immunohistochemistry, showing LH and αGSU expression in ovine PT and pars distalis (PD). Scale bars, 500 μm. B, Double immunofluorescence, showing expression of LH, αGSU, and merged images in ovine PT and PD. Scale bars, 20 μm. C, Double immunofluorescence, showing expression of αGSU and NPAS4 in ovine PT. Scale bars, 20 μm. PN, pars nervosa; III v, third ventricle.
Figure 3.
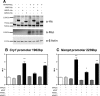
NPAS4 Activates Cry1 and Nampt Promoter Activity in the Presence of Heterodimeric Partner Proteins. A, Western blots on total proteins from COS7 cells transfected with empty Myc and/or His expression vectors and vectors expressing the full-length ovine NPAS4-Myc, ARNT-His, ARNTL-His, or ARNT2-His. B and C, In vitro transfection of COS7 cells with a fragment of the ovine Cry1 1902 bp (B, −1805 +97 relative to TSS) or Nampt 2239bp (C, −1845 +394 relative to putative TSS) promoter driving the expression of the luciferase gene, together with empty Myc and/or His expression vectors and vectors expressing the full-length ovine NPAS4, ARNT, ARNTL, and/or ARNT2. Results show relative biolumnescence (RLU), after 24 hours, in each group (n = 4 replicates per group) normalized to control group (empty vectors), from 1 of 3 representative experiments. Statistical differences between groups were analyzed after 1-way ANOVA followed by Bonferrroni's post hoc test (**, P ≤ .01; ****, P ≤ .0001 against control group).
Figure 4.
Mutational Analysis of CMEs of the 313-bp Ovine Cry1 Promoter. A, Localization of the 3 putative CMEs (N(G/A)CGTG, CME1, CME2 and CME3) identified in the conserved 313-bp proximal promoter region (position −216 +97 relative to TSS) of the ovine Cry1 promoter (TSS). Nucleotide sequence alignment and site-directed mutagenesis alterations shown below. B–I, In vitro transfection of COS7 cells with a 313-bp fragment of the ovine Cry1 promoter driving the expression of the luciferase gene, together with empty Myc and/or His expression vectors and vectors expressing the full-length ovine NPAS4, ARNT, ARNTL, and/or ARNT2. Results show relative biolumnescence (RLU) after 24 hours, in each group (n = 4 replicates per group) normalized to control group (empty vectors), from 1 of 3 representative experiments. Statistical differences between groups were analyzed after 1-way ANOVA followed by Bonferroni's post hoc test (**, P ≤ .01; ***, P ≤ .001; ****, P ≤ .0001 against control group).
Figure 5.
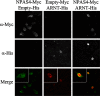
Cellular Localization of NPAS4 and ARNT in Transfected COS7 Cells. Immunocytochemistry of COS7 cells overexpressing NPAS4-Myc or/and ARNT-His. Images show staining obtained with the anti-Myc only (top panels), anti-His only (middle panels), and combined (bottom panels).
Similar articles
- Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: evidence for an internal coincidence timer.
Lincoln G, Messager S, Andersson H, Hazlerigg D. Lincoln G, et al. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13890-5. doi: 10.1073/pnas.212517599. Epub 2002 Oct 8. Proc Natl Acad Sci U S A. 2002. PMID: 12374857 Free PMC article. - Clock genes and the long-term regulation of prolactin secretion: evidence for a photoperiod/circannual timer in the pars tuberalis.
Lincoln GA, Andersson H, Hazlerigg D. Lincoln GA, et al. J Neuroendocrinol. 2003 Apr;15(4):390-7. doi: 10.1046/j.1365-2826.2003.00990.x. J Neuroendocrinol. 2003. PMID: 12622839 Review. - Regulation of the ovine MT1 melatonin receptor promoter: interaction between multiple pituitary transcription factors at different phases of development.
Johnston JD, Schuster C, Barrett P, Hazlerigg DG. Johnston JD, et al. Mol Cell Endocrinol. 2007 Mar 30;268(1-2):59-66. doi: 10.1016/j.mce.2007.01.015. Epub 2007 Feb 1. Mol Cell Endocrinol. 2007. PMID: 17337323 - Melatonin receptor 1-dependent gene expression in the mouse pars tuberalis as revealed by cDNA microarray analysis and in situ hybridization.
Unfried C, Burbach G, Korf HW, von Gall C. Unfried C, et al. J Pineal Res. 2010 Mar;48(2):148-56. doi: 10.1111/j.1600-079X.2009.00738.x. Epub 2010 Jan 8. J Pineal Res. 2010. PMID: 20070488 - Clock genes in calendar cells as the basis of annual timekeeping in mammals--a unifying hypothesis.
Lincoln GA, Andersson H, Loudon A. Lincoln GA, et al. J Endocrinol. 2003 Oct;179(1):1-13. doi: 10.1677/joe.0.1790001. J Endocrinol. 2003. PMID: 14529560 Review.
Cited by
- Binary Switching of Calendar Cells in the Pituitary Defines the Phase of the Circannual Cycle in Mammals.
Wood SH, Christian HC, Miedzinska K, Saer BR, Johnson M, Paton B, Yu L, McNeilly J, Davis JR, McNeilly AS, Burt DW, Loudon AS. Wood SH, et al. Curr Biol. 2015 Oct 19;25(20):2651-62. doi: 10.1016/j.cub.2015.09.014. Epub 2015 Sep 24. Curr Biol. 2015. PMID: 26412130 Free PMC article. - Melatonin receptors: molecular pharmacology and signalling in the context of system bias.
Cecon E, Oishi A, Jockers R. Cecon E, et al. Br J Pharmacol. 2018 Aug;175(16):3263-3280. doi: 10.1111/bph.13950. Epub 2017 Aug 17. Br J Pharmacol. 2018. PMID: 28707298 Free PMC article. Review. - Clocks for all seasons: unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary.
Wood S, Loudon A. Wood S, et al. J Endocrinol. 2014 Aug;222(2):R39-59. doi: 10.1530/JOE-14-0141. Epub 2014 Jun 2. J Endocrinol. 2014. PMID: 24891434 Free PMC article. Review. - Genome-Wide Association Study Demonstrates the Role Played by the CD226 Gene in Rasa Aragonesa Sheep Reproductive Seasonality.
Lakhssassi K, Lahoz B, Sarto P, Iguácel LP, Folch J, Alabart JL, Serrano M, Calvo JH. Lakhssassi K, et al. Animals (Basel). 2021 Apr 19;11(4):1171. doi: 10.3390/ani11041171. Animals (Basel). 2021. PMID: 33921837 Free PMC article. - Genomic Decoding of Neuronal Depolarization by Stimulus-Specific NPAS4 Heterodimers.
Brigidi GS, Hayes MGB, Delos Santos NP, Hartzell AL, Texari L, Lin PA, Bartlett A, Ecker JR, Benner C, Heinz S, Bloodgood BL. Brigidi GS, et al. Cell. 2019 Oct 3;179(2):373-391.e27. doi: 10.1016/j.cell.2019.09.004. Cell. 2019. PMID: 31585079 Free PMC article. Retracted.
References
- Hazlerigg D, Loudon A. New insights into ancient seasonal life timers. Curr Biol. 2008;18:R795–R804 - PubMed
- Lincoln GA, Short RV. Seasonal breeding: nature's contraceptive. Recent Prog Horm Res. 1980;36:1–52 - PubMed
- Dupré SM. Encoding and decoding photoperiod in the mammalian pars tuberalis. Neuroendocrinology. 2011;94:101–112 - PubMed
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190 - PubMed
- Hanon EA, Lincoln GA, Fustin JM, et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/G003033/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G1100355/MRC_/Medical Research Council/United Kingdom
- BB/J004235/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004316/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB G003033/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous