Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo - PubMed (original) (raw)
Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo
S Yasuda et al. Clin Exp Immunol. 2013 Jun.
Abstract
Recent basic and clinical studies have shown that the programmed death ligand (PD-L)/PD-1 pathway has a significant role in tumour immunity, and its blockade has a therapeutic potential against several human cancers. We hypothesized that anti-angiogeneic treatment might augment the efficacy of PD-1 blockade. To this end, we evaluated combining the blockade of PD-1 and vascular endothelial growth factor receptor 2 (VEGFR2) in a murine cancer model using Colon-26 adenocarcinoma. Interestingly, simultaneous treatment with anti-PD-1 and anti-VEGFR2 monoclonal antibodies (mAbs) inhibited tumour growth synergistically in vivo without overt toxicity. Blocking VEGFR2 inhibited tumour neovascularization significantly, as demonstrated by the reduced number of microvessels, while PD-1 blockade had no impact on tumour angiogenesis. PD-1 blockade might promote T cell infiltration into tumours and significantly enhanced local immune activation, as shown by the up-regulation of several proinflammatory cytokine expressions. Importantly, VEGFR2 blockade did not interfere with T cell infiltration and immunological activation induced by PD-1 blockade. In conclusion, simultaneous blockade of PD-1 and VEGFR2 induced a synergistic in-vivo anti-tumour effect, possibly through different mechanisms that might not be mutually exclusive. This unique therapeutic strategy may hold significant promise for future clinical application.
© 2013 British Society for Immunology.
Figures
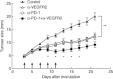
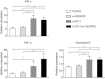
Fig. 1
Simultaneous blockade of programmed death (PD)-1 and vascular endothelial growth factor receptor 2 (VEGFR2) induced synergistic anti-tumour effect in vivo. BALB/c mice were inoculated subcutaneously with Colon-26 cells and were given with control rat immunoglobulin (Ig)G, anti-PD-1 monoclonal antibody (mAb), anti-VEGFR2 mAb or both mAbs five times (arrow). Data are presented as mean ±standard error of seven to 10 mice of each group. *P < 0·05; **P < 0·01.
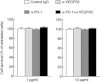
Fig. 2
Programmed death (PD)-1and vascular endothelial growth factor receptor 2 (VEGFR2) blockade did not have any direct effect on cancer cell growth in vitro. A total of 3000 Colon-26 cells were co-cultured with anti-PD-1 monoclonal antibody (mAb), anti-VEGFR2 mAb or control rat immunoglobulin (Ig)G for 72 h, and cell proliferation was determined by MTS assay.
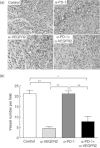
Fig. 3
Treatment with anti-vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody (mAb) inhibited tumour neovascularization. (a) Immunohistochemistry analysis by staining with CD34. Representative tumours from mice treated with control rat IgG, anti-programmed death (PD)-1 mAb, anti-VEGFR2 mAb or both mAbs. (b) Tumour microvessels were counted at ×200 magnification. Data are collected from four to seven mice of each group. *P < 0·01; **P < 0·001.
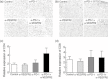
Fig. 4
(a) Immunohistochemical staining of CD4+ and (b) CD8+ T cells in tumour tissue at day 11. Representative pictures of mice for each treatment are shown. Programmed death (PD)-1 blockade and combination treatment seemed to induce more CD4+ and CD8+ T cell infiltration compared to control and vascular endothelial growth factor receptor 2 (VEGFR2) blockade. (c) Quantification of tumour-infiltrating CD4+ and (d) CD8+ T cells by real-time polymerase chain reaction (PCR). There was a tendency towards increased T cell infiltration by the treatment of anti-PD-1 monoclonal antibody (mAb) and combination treatment. Anti-VEGFR2 mAb treatment did not interfere with T cell infiltration. Data are collected from four to seven mice of each group.
Fig. 5
Expression of interferon (IFN)-γ, tumour necrosis factor (TNF)-α and granzyme B was up-regulated significantly by anti-programmed death (PD)-1 monoclonal antibody (mAb) or combination mAb treatment compared with control. Treatment of anti-VEGFR2 mAb alone did not increase each cytokine expression. Data are collected from four to seven mice of each group. *P < 0·05.
Similar articles
- Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma.
Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, Kikuchi H, Mamessier E, Aoki S, Ramjiawan RR, Ochiai H, Bardeesy N, Huang P, Cobbold M, Zhu AX, Jain RK, Duda DG. Shigeta K, et al. Hepatology. 2020 Apr;71(4):1247-1261. doi: 10.1002/hep.30889. Epub 2019 Oct 14. Hepatology. 2020. PMID: 31378984 Free PMC article. - Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade.
Li Q, Wang Y, Jia W, Deng H, Li G, Deng W, Chen J, Kim BYS, Jiang W, Liu Q, Liu J. Li Q, et al. Clin Cancer Res. 2020 Apr 1;26(7):1712-1724. doi: 10.1158/1078-0432.CCR-19-2179. Epub 2019 Dec 17. Clin Cancer Res. 2020. PMID: 31848190 Clinical Trial. - Apatinib potentiates the therapeutic effect of anti-PD-1 in locally advanced head and neck cancers.
Liu S, Zhang L, Ye W, Zhou R, Gu Z, Shi C, Xu S, Li J, Zhang Z, Han Y. Liu S, et al. Oral Dis. 2024 Jul;30(5):2940-2951. doi: 10.1111/odi.14768. Epub 2023 Oct 16. Oral Dis. 2024. PMID: 37846172 - Anti-VEGF/VEGFR2 Monoclonal Antibodies and their Combinations with PD-1/PD-L1 Inhibitors in Clinic.
Gao F, Yang C. Gao F, et al. Curr Cancer Drug Targets. 2020;20(1):3-18. doi: 10.2174/1568009619666191114110359. Curr Cancer Drug Targets. 2020. PMID: 31729943 Review. - Combined blockade of vascular endothelial growth factor and programmed death 1 pathways in advanced kidney cancer.
Einstein DJ, McDermott DF. Einstein DJ, et al. Clin Adv Hematol Oncol. 2017 Jun;15(6):478-488. Clin Adv Hematol Oncol. 2017. PMID: 28749908 Review.
Cited by
- Immune Checkpoint Inhibitors as Monotherapy or Within a Combinatorial Strategy in Advanced Hepatocellular Carcinoma.
Guardascione M, Toffoli G. Guardascione M, et al. Int J Mol Sci. 2020 Aug 31;21(17):6302. doi: 10.3390/ijms21176302. Int J Mol Sci. 2020. PMID: 32878115 Free PMC article. Review. - Combinations of Anti-Angiogenic Agents and Immune Checkpoint Inhibitors in Renal Cell Carcinoma: Best Option?
Granet-Vaissiere E, Lefort F, Domblides C, Larroquette M, Ravaud A, Bernhard JC, Gross-Goupil M. Granet-Vaissiere E, et al. Cancers (Basel). 2023 Feb 7;15(4):1048. doi: 10.3390/cancers15041048. Cancers (Basel). 2023. PMID: 36831392 Free PMC article. Review. - Antiangiogenic antibody BD0801 combined with immune checkpoint inhibitors achieves synergistic antitumor activity and affects the tumor microenvironment.
Xue L, Gao X, Zhang H, Tang J, Wang Q, Li F, Li X, Yu X, Lu Z, Huang Y, Tang R, Yang W. Xue L, et al. BMC Cancer. 2021 Oct 22;21(1):1134. doi: 10.1186/s12885-021-08859-5. BMC Cancer. 2021. PMID: 34686154 Free PMC article. - Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients.
Shiono A, Kaira K, Mouri A, Yamaguchi O, Hashimoto K, Uchida T, Miura Y, Nishihara F, Murayama Y, Kobayashi K, Kagamu H. Shiono A, et al. Thorac Cancer. 2019 Apr;10(4):775-781. doi: 10.1111/1759-7714.12998. Epub 2019 Feb 27. Thorac Cancer. 2019. PMID: 30809973 Free PMC article. - Combining Immune Checkpoint Inhibitors with Anti-Angiogenic Agents.
Ciciola P, Cascetta P, Bianco C, Formisano L, Bianco R. Ciciola P, et al. J Clin Med. 2020 Mar 3;9(3):675. doi: 10.3390/jcm9030675. J Clin Med. 2020. PMID: 32138216 Free PMC article. Review.
References
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. - PubMed
- Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. - PubMed
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. - PubMed
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials