Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes - PubMed (original) (raw)
. 2013 Apr 18;38(4):792-804.
doi: 10.1016/j.immuni.2013.04.004.
Andrew Chow, Clara Noizat, Pearline Teo, Mary Beth Beasley, Marylene Leboeuf, Christian D Becker, Peter See, Jeremy Price, Daniel Lucas, Melanie Greter, Arthur Mortha, Scott W Boyer, E Camilla Forsberg, Masato Tanaka, Nico van Rooijen, Adolfo García-Sastre, E Richard Stanley, Florent Ginhoux, Paul S Frenette, Miriam Merad
Affiliations
- PMID: 23601688
- PMCID: PMC3853406
- DOI: 10.1016/j.immuni.2013.04.004
Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes
Daigo Hashimoto et al. Immunity. 2013.
Abstract
Despite accumulating evidence suggesting local self-maintenance of tissue macrophages in the steady state, the dogma remains that tissue macrophages derive from monocytes. Using parabiosis and fate-mapping approaches, we confirmed that monocytes do not show significant contribution to tissue macrophages in the steady state. Similarly, we found that after depletion of lung macrophages, the majority of repopulation occurred by stochastic cellular proliferation in situ in a macrophage colony-stimulating factor (M-Csf)- and granulocyte macrophage (GM)-CSF-dependent manner but independently of interleukin-4. We also found that after bone marrow transplantation, host macrophages retained the capacity to expand when the development of donor macrophages was compromised. Expansion of host macrophages was functional and prevented the development of alveolar proteinosis in mice transplanted with GM-Csf-receptor-deficient progenitors. Collectively, these results indicate that tissue-resident macrophages and circulating monocytes should be classified as mononuclear phagocyte lineages that are independently maintained in the steady state.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
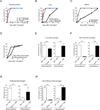
Figure 1. Fate mapping reveals that tissue resident macrophages are maintained independently of adult hematopoiesis
(A-F) The percentage of tdTomato+ cells among purified hematopoietic progenitors (A, D, open circles), monocytes (B, E, gray circles), and tissue resident macrophages (C, F, black filled circles) in _Mx1cre_×R26Tomato (A-C) and _S100a4cre_×R26Tomato (D-F) are shown. Representative data from two separate experiments are shown. (G, H) The percentage of GFP+ cells among monocytes (G, gray circles), and tissue resident macrophages (H, black circles) in _Flk2cre_×R26Tomato/GFP mice are shown. Data were pooled from two independent experiments. Dashed lines in C, F, H indicate % recombination in blood (C, F) or spleen (H) Gr1high monocytes. LT-HSC: long-term hematopoietic stem cells; MPP: multipotent hematopoietic progenitors; CMP: common myeloid progenitors; GMP: granulocyte macrophage progenitor; MDP: macrophage dendritic cell progenitor. *: P < 0.05; **: P < 0.01; ***: P < 0.001 compared to Gr1high monocytes. See also Figure S1.
Figure 2. Parabiosis studies indicate that tissue resident macrophages are maintained independently of monocytes
(A, B) C57BL/6 CD45.2+ and CD45.1+ parabionts were surgically connected for 2 months (A) or 5 months (B). Bars show the percentage non-host cells among peripheral blood monocytes (white bars) and tissue resident macrophages (black bars) in host parabionts (n=4–6). Representative data from two separate experiments are shown as mean +/− SEM. *: P < 0.05; **: P < 0.01; ***: P < 0.001 compared to Gr1high monocytes. (C) Parabiotic pairs were generated between wild-type CD45.1+ mice and Ccr2−/− CD45.2+ mice. The percentage of non-host cells in the CD45.2+ mouse among peripheral blood monocytes (open circles) and tissue macrophages (filled circles) were analyzed 2 months after surgery. (D) C57BL/6 mice were pulsed with BrdU for 3 weeks (1mg i.p. daily) and BrdU incorporation in tissue macrophages was assessed 1 day (Day 0, open circles) and 22 days (Day 21, filled circles) after the last pulse. *: P < 0.05. See also Figure S2.
Figure 3. Tissue resident macrophages repopulate locally independently of CCR2+ progenitors
(A) CD169DTR/+ mice were injected i.p. with 10 µg/kg DT (n=5, filled bars) or control diluent (n=3, open bars) on day 1 and 4 and the absolute numbers of lung macrophages were enumerated 2 days and 9 days after the last injection. Representative data from three separate experiments are shown as mean +/− SEM. **: P < 0.01. (B, C) CD169DTR/+×Ccr2+/− (open circles, n=5/time point), and CD169DTR/+×Ccr2−/− mice (filled circles, n=6/time point) were injected with DT on day 0 and the number of BM macrophages (B) and lung macrophages (C) were analyzed at 2, 9, and 16 days after DT administration. CD169+/+×Ccr2+/− (open triangle) and CD169+/+×Ccr2−/− (filled triangle) mice treated with DT were used as non-depleted controls. The absolute cell numbers are represented as relative values with the mean absolute number of lung macrophages in non-depleted CD169+/+×Ccr2+/− mice set at 100%. Data were pooled from two independent experiments and shown as mean +/− SEM. (D-E) CD169DTR/+ (CD45.2+, n=5) mice were infused with 7.5 × 107 congenic CD45.1+ BM cells, followed by DT (filled circles) or PBS (open circles) administration on days 4 and 7 after BM cell transfer. The percentage of donor-derived CD45.1+ cells among (D) BM progenitor cells (white bars) and blood monocytes (Mono, gray bars) and (E) lung monocytes and tissue macrophages were analyzed sixteen days after the transfer. Representative data from three independent experiments are shown as mean +/− SEM. *: P < 0.05; **: P < 0.01 compared to Gr1high monocytes. (F-H) S100a4Cre×R26Tomato mice were infected intranasally with 100 pfu PR8 influenza virus (F and H filled circles) or instilled intranasally with 50µg Poly (I:C) (G and H gray circles) or control PBS (F-H, open circles). Kinetics of lung macrophages number at different times after PR8 infection (F) or Poly (I:C) injection (G) and numbers of tdTomato− lung macrophages at six days and four weeks following PR8 infection or Poly (I:C) injection are shown (H). Data are represented as mean +/− SEM. See also Figure S3.
Figure 4. Repopulation of lung tissue resident macrophages is dependent on local cytokine production
(A, B) Wild-type or CD169DTR/+ mice were administered 10µg/kg DT on days −5 and −2 and injected i.p. with 1mg BrdU /mouse on day −1 prior to analysis. Flow cytometric plots depict percentage of BrdU positive cells among total lung macrophages in non-BrdU injected mice (left panel), WT mice treated with DT (center panel), or CD169DTR/+ mice treated with DT (right panel). (B, C) Quantitation of the percentage of BrdU+ cells among lung macrophages (B) and BM macrophages (C) in WT (open circles) and CD169DTR/+ (filled circles) mice treated with DT. (D-H) mRNA was purified from lung homogenates and expression of IL-4 (D), IL-13 (E), CSF-1 (F), CSF-2 (G), and Flt3L (H) were quantified with real-time PCR relative to the expression of GAPDH. (I) CD169DTR/+ mice were injected with PBS or DT on days −12 and −9 prior to analysis followed by i.n. injection of 600µg of anti-Csf-1R Ab (clone: AFS98) on days −8, −6, −4, and −1 and 100µg of anti-Csf-2 neutralizing Ab (clone: MP122E9) on days −7, −5, −2 prior to the analysis. The numbers of lung tissue resident macrophages in mice that received rat IgG alone (open bar, n=16), anti-Csf-1R mAb and Csf-2 mAb blockade without DT (hatched bar, n=5), DT + control rat IgG (gray filled bar, n=9), and DT in addition to anti-Csf-1R mAb and Csf-2 mAb blockade (black filled bar, n=10) are shown. Data were pooled from three independent experiments and shown as mean +/− SEM. (J, K) CD169DTR/+ mice were treated with 10 εg/kg DT on day −16, pulsed with 1mg/day BrdU on days −15 through −5, and re-injected with DT (black bars, n=8) or PBS (white bars, n=8) on day −2 prior to analysis. Mice were sacrificed on day 0 and BrdU incorporation and Ki67 expression were analyzed using flow cytometry. Representative FACS plots of Ki67 and BrdU staining in lung macrophages (J) are shown. Ki67 expression (cycling cells) among BrdU positive fraction and BrdU negative fraction of lung macrophages were quantified (K). Data were pooled from two independent experiments and shown as mean +/− SEM. *: P < 0.05; **: P < 0.01; ***: P < 0.001. See also Figure S4.
Figure 5. X-ray irradiation does not abrogate host tissue macrophage repopulation potential
(A-D) C57BL/6 CD45.2+ mice were lethally irradiated and transplanted with 5 × 106 congenic BM CD45.1+ cells. The percentage of donor (CD45.1+) cells among circulating monocytes and CNS microglia (A), monocytes and/or macrophages in the lung (B), spleen (C), BM (D), and peritoneum (D) are shown (n=3–4/time points). (E-H) C57BL/6 CD45.1+ mice were lethally irradiated and transplanted with 1 × 106 fetal liver cells isolated from C57BL/6 CD45.2+ Csf-1r−/− (black bars, n=7) mice or control littermate (white bars, n=7). The absolute numbers of host and donor tissue resident macrophages in the lung (E), spleen (F), peritoneum (G), and BM (H) were enumerated 4 months after transplant. Data were pooled from two independent experiments and shown as mean +/− SEM. **: P < 0.01; ***: P < 0.001. See also Figure S5.
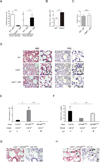
Figure 6. Lung macrophages repopulate locally after lethal irradiation and promote lung tissue integrity
(A-D) CD45.1+ mice were lethally irradiated and transplanted with 5 × 106 BM cells isolated from CD45.2+ Csf-2r−/− (black bars, n=6) mice or control CD45.2+ littermate (white bars, n=5). (A) The absolute numbers of donor- and host-derived lung macrophages in right lung lobes were enumerated 2 months after bone marrow transplantation. (B, C) Protein concentrations in the bronchial alveolar lavage fluid (BALF) were quantified in wild-type mice (white bar), Csf-2r−/− mice (black bar), and wild-type mice transplanted with wild-type (gray bar) or Csf-2r−/− (hatched bar) BM (n=3/group) two months after bone marrow transplantation. Data were pooled from two independent experiments and shown as mean +/− SEM. (D) Representative sections of left lung lobes stained with H&E (left) and PAS (right) obtained from steady-state wild-type (WT) mice (top), steady-state Csf-2r−/− mice (center), and WT mice transplanted with Csf-2r−/− (bottom) BM. Arrow heads: PAS+ eosinophilic material within the alveolar spaces. Scale bar: 25µm. (E-H) Csf-2r−/− mice and CD169DTR/+ mice were lethally irradiated and reconstituted with 5 × 106 BM cells isolated from C57BL/6 WT or Csf-2r−/− mice. A group of CD169DTR/+Csf-2r+/+ recipients were injected with DT (10µg/kg, twice weekly) starting from day +3 post-transplant. The absolute numbers of macrophages in the right lung lobes (E) and protein concentrations in BALF (F) in [Csf-2r−/− into _Csf-2r−/−_] (black bars, n=3) and [Csf-2r−/− into CD169DTR/+] chimera treated with PBS (gray bars, n=6) or DT (hatched bars, n=6) were enumerated two months after transplant. Data were pooled from two independent experiments and shown as mean +/− SEM. Paraffin sections of the left lung lobes of recipients of [Csf-2r−/− into CD169DTR/+] chimeras treated with PBS (G) or DT (H) at 2 months post transplant were stained with H&E (left panel) and PAS stain (right panel). Sections isolated from [Csf-2r−/− into CD169DTR/+] chimera treated with DT (H) demonstrate granular eosinophilic material positive for PAS within the alveolar spaces (arrow heads) whereas sections from [Csf-2r−/− into CD169DTR/+] chimera treated with diluent (G) demonstrate normal alveolar structure. Scale bars: 25 µm. See also Figure S6. *: P < 0.05; **: P < 0.01.
Similar articles
- The pulmonary alveolar proteinosis in granulocyte macrophage colony-stimulating factor/interleukins 3/5 beta c receptor-deficient mice is reversed by bone marrow transplantation.
Nishinakamura R, Wiler R, Dirksen U, Morikawa Y, Arai K, Miyajima A, Burdach S, Murray R. Nishinakamura R, et al. J Exp Med. 1996 Jun 1;183(6):2657-62. doi: 10.1084/jem.183.6.2657. J Exp Med. 1996. PMID: 8676086 Free PMC article. - Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF.
Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Guilliams M, et al. J Exp Med. 2013 Sep 23;210(10):1977-92. doi: 10.1084/jem.20131199. Epub 2013 Sep 16. J Exp Med. 2013. PMID: 24043763 Free PMC article. - Homeostasis in the mononuclear phagocyte system.
Jenkins SJ, Hume DA. Jenkins SJ, et al. Trends Immunol. 2014 Aug;35(8):358-67. doi: 10.1016/j.it.2014.06.006. Epub 2014 Jul 18. Trends Immunol. 2014. PMID: 25047416 Review. - Regulation of myeloid development and function by colony stimulating factors.
Barreda DR, Hanington PC, Belosevic M. Barreda DR, et al. Dev Comp Immunol. 2004 May 3;28(5):509-54. doi: 10.1016/j.dci.2003.09.010. Dev Comp Immunol. 2004. PMID: 15062647 Review.
Cited by
- Alternations in inflammatory macrophage niche drive phenotypic and functional plasticity of Kupffer cells.
Huang HY, Chen YZ, Zhao C, Zheng XN, Yu K, Yue JX, Ju HQ, Shi YX, Tian L. Huang HY, et al. Nat Commun. 2024 Oct 29;15(1):9337. doi: 10.1038/s41467-024-53659-7. Nat Commun. 2024. PMID: 39472435 Free PMC article. - Macrophages contribute to the cyclic activation of adult hair follicle stem cells.
Castellana D, Paus R, Perez-Moreno M. Castellana D, et al. PLoS Biol. 2014 Dec 23;12(12):e1002002. doi: 10.1371/journal.pbio.1002002. eCollection 2014 Dec. PLoS Biol. 2014. PMID: 25536657 Free PMC article. - Harnessing and Enhancing Macrophage Phagocytosis for Cancer Therapy.
Chen S, Lai SWT, Brown CE, Feng M. Chen S, et al. Front Immunol. 2021 Mar 10;12:635173. doi: 10.3389/fimmu.2021.635173. eCollection 2021. Front Immunol. 2021. PMID: 33790906 Free PMC article. Review. - How cell migration helps immune sentinels.
Delgado MG, Lennon-Duménil AM. Delgado MG, et al. Front Cell Dev Biol. 2022 Oct 4;10:932472. doi: 10.3389/fcell.2022.932472. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36268510 Free PMC article. Review. - Functional heterogeneity of alveolar macrophage population based on expression of CXCL2.
Xu-Vanpala S, Deerhake ME, Wheaton JD, Parker ME, Juvvadi PR, MacIver N, Ciofani M, Shinohara ML. Xu-Vanpala S, et al. Sci Immunol. 2020 Aug 7;5(50):eaba7350. doi: 10.1126/sciimmunol.aba7350. Sci Immunol. 2020. PMID: 32769172 Free PMC article.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self -renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. - PubMed
- Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M. CD169-Positive Macrophages Dominate Antitumor Immunity by Crosspresenting Dead Cell-Associated Antigens. Immunity. 2011;34:85–95. - PubMed
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2T32GM008646/GM/NIGMS NIH HHS/United States
- HL116340/HL/NHLBI NIH HHS/United States
- R01 HL086899/HL/NHLBI NIH HHS/United States
- R01 HL116340/HL/NHLBI NIH HHS/United States
- CA154947A/CA/NCI NIH HHS/United States
- HL069438/HL/NHLBI NIH HHS/United States
- F30 HL099028/HL/NHLBI NIH HHS/United States
- R01 DK056638/DK/NIDDK NIH HHS/United States
- R01 CA173861/CA/NCI NIH HHS/United States
- U19 AI089987/AI/NIAID NIH HHS/United States
- R01 HL069438/HL/NHLBI NIH HHS/United States
- R01 CA154947/CA/NCI NIH HHS/United States
- DK056638/DK/NIDDK NIH HHS/United States
- T32 GM008646/GM/NIGMS NIH HHS/United States
- 5F30HL099028/HL/NHLBI NIH HHS/United States
- HL097700/HL/NHLBI NIH HHS/United States
- R01 HL097700/HL/NHLBI NIH HHS/United States
- AI10008/AI/NIAID NIH HHS/United States
- AI089987/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials