A phenotypic change but not proliferation underlies glial responses in Alzheimer disease - PubMed (original) (raw)
A phenotypic change but not proliferation underlies glial responses in Alzheimer disease
Alberto Serrano-Pozo et al. Am J Pathol. 2013 Jun.
Abstract
Classical immunohistochemical studies in the Alzheimer disease (AD) brain reveal prominent glial reactions, but whether this pathological feature is due primarily to cell proliferation or to a phenotypic change of existing resting cells remains controversial. We performed double-fluorescence immunohistochemical studies of astrocytes and microglia, followed by unbiased stereology-based quantitation in temporal cortex of 40 AD patients and 32 age-matched nondemented subjects. Glial fibrillary acidic protein (GFAP) and major histocompatibility complex II (MHC2) were used as markers of astrocytic and microglial activation, respectively. Aldehyde dehydrogenase 1 L1 and glutamine synthetase were used as constitutive astrocytic markers, and ionized calcium-binding adaptor molecule 1 (IBA1) as a constitutive microglial marker. As expected, AD patients had higher numbers of GFAP(+) astrocytes and MHC2(+) microglia than the nondemented subjects. However, both groups had similar numbers of total astrocytes and microglia and, in the AD group, these total numbers remained essentially constant over the clinical course of the disease. The GFAP immunoreactivity of astrocytes, but not the MHC2 immunoreactivity of microglia, increased in parallel with the duration of the clinical illness in the AD group. Cortical atrophy contributed to the perception of increased glia density. We conclude that a phenotypic change of existing glial cells, rather than a marked proliferation of glial precursors, accounts for the majority of the glial responses observed in the AD brain.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
Confocal images representative of double-fluorescence immunohistochemical study of astrocytes with ALDH1L1 (green) (A–D) and GFAP (red) (E–H); merged images are also shown (I–L). Representative images from four cases are shown: one nondemented control subject (A, E, and I), and three AD patients with 5 years (B, F, and J), 12 years (C, G, and K), and 19 years (D, H, and L) of clinical illness. The majority of astrocytes in the nondemented individual were GFAP− (resting astrocytes), as seen in the merged image (I), whereas astrocytes in the AD cortex displayed a reactive phenotype with GFAP-immunoreactivity that was associated with the duration of the illness (J, K, and L). Disease-related cortical atrophy produced an increase in the density of astrocytes (green) (A versus D). Scale bar = 50 μm.
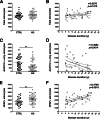
Figure 2
Stereology-based quantitative analysis of astrocytes double-labeled for ALDH1L1 and GFAP. The total number of astrocytes did not differ between AD patients and nondemented control (CTRL) subjects (A) and remained essentially constant through the disease clinical course (B). The number of ALDH1L1+GFAP+ astrocytes was higher in the AD group (E), whereas the number of ALDH1L1+GFAP− astrocytes was higher in the nondemented group (C). Astrocytes became increasingly reactive (GFAP+) as the clinical phase of the disease advances (D and F). Counts in each case are not density measures, but estimates of the number of cells per 1 cm-long full-width cortex from a single section of temporal isocortex. Data are expressed as individual values with means ± SEM (A, C, and E) or 95% confidence interval of the mean slope (B, D, and F). ∗∗P < 0.01.
Figure 3
Confocal images of the double-fluorescence immunohistochemical study of astrocytes with GS (green) (A, E, and I) and GFAP (red) (B, F, and J). In merged images (C, G, and K), the boxed region is shown at higher magnification in the next panel (D, H, L). Representative images from three cases are shown: one nondemented control subject (A–D) and two AD patients with 5 years (E–H) and 19 years (I–L) of clinical illness. Many astrocytes in nondemented subjects were positive only for GS (C and D), whereas astrocytes in AD patients exhibited an increased immunoreactivity for GFAP that was associated with the progression of the disease (G, H, K, and L). Disease-related cortical atrophy produced an increase in the density of astrocytes (eg, A versus I). Scale bars: 50 μm (A–C, E–G, and I–K); 20 μm (D, H, and L).
Figure 4
Stereology-based quantitative analysis of astrocytes double-labeled for GS and GFAP. The number of GS+GFAP+ astrocytes was higher in the AD group (E), whereas the number of GS+GFAP− astrocytes was higher in the nondemented group (C), but the number of total astrocytes did not differ significantly between AD patients and nondemented subjects (A). This phenotypic change paralleled the progression of the disease, but the number of total astrocytes remained essentially constant through its clinical course (B, D, and F). Counts in each case are not density measures, but estimates of the number of cells per 1 cm-long full-width cortex from a single section of temporal isocortex. Data are expressed as individual values with means ± SEM (A, C, and E) or 95% confidence interval of the mean slope (B, D, and F). ∗P < 0.0001.
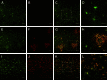
Figure 5
Confocal images of the double-fluorescence immunohistochemical study of microglia with IBA1 (green) (A and E) and MHC2 (red) (B and F). Representative images from a nondemented subject (A–D) and an AD patient (E–H) are shown. Also shown are merged high-power images from two different nondemented individuals (I and J) and from two different AD patients (K and L). Thioflavin-S staining (blue-green) identifies plaques (G, H, K, and L). The uniform morphology and labeling of microglia in nondemented subjects contrasts with the morphological and molecular diversity of microglia in AD. Three subpopulations of microglial cells are discernible with this double labeling: IBA1+MHC2− (arrows), IBA1+MHC2+ (arrowheads), and IBA1−MHC2+ (double arrowheads) cells. Scale bars: 50 μm (A–H); 20 μm (K and L); 10 μm (I and J).
Figure 6
Stereology-based quantitative analysis of microglia double-labeled for IBA1 and MHC2. The total number of microglia did not differ significantly between the nondemented and the AD groups (A). Within the AD group, neither total microglial cells (B) nor any of the microglia subtypes (D, F, and H) was observed to increase with the clinical progression of the disease. However, IBA1+MHC2− microglia (C) was significantly more abundant in the nondemented group, and IBA1−MHC2+ microglia (G) were significantly more abundant in the AD. There was a trend toward increase in IBA1+MHC2+ microglia in the AD group (E). Counts in each case are not density measures, but estimates of the number of cells per 1 cm-long full-width cortex from a single section of temporal isocortex. Data are expressed as individual values with means ± SEM (A, C, E, and G) or 95% confidence interval of the mean slope (B, D, F, and H).∗P < 0.05, †P < 0.0001.
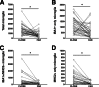
Figure 7
Association of the three microglial subpopulations with dense-core plaques and NFTs. The randomly selected microglial cells were categorized as close if located within 50 μm from the closest dense-core plaque or NFT, or as far if located more than 50 μm away from it. The three subsets of microglial cells are similarly attracted by the pathological hallmarks of AD and recruited toward them. Counts in each case are not density measures, but estimates of the number of cells per 1 cm-long full-width cortex from a single section of temporal isocortex. Counts close and far (from AD pathological hallmarks) from each of the 40 AD subjects are connected with lines. ∗P < 0.0001.
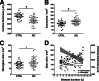
Figure 8
Effect of the APOE ɛ4 allele on microglial phenotype. Numbers of total microglia (A), IBA1+MHC2− microglia (B), and IBA1−MHC2+ microglia (D) did not differ between APOE ɛ4 carriers and noncarriers. Only for IBA1+MHC2+ microglia (C) were numbers significantly higher in APOE ɛ4 carriers, compared with noncarriers. Counts in each case are not density measures, but estimates of the number of cells per 1 cm-long full-width cortex from a single section of temporal isocortex. Data are expressed as individual values with means ± SEM. ∗P < 0.01.
Figure 9
Effect of cortical atrophy on density measures of astrocytes and microglia. A: As expected, the quantification of cortical thickness of the temporal isocortex (Brodmann area 38) in the study subjects revealed significant cortical atrophy in the AD group, compared with the nondemented group. B and C: Unlike total numbers (Figures 2, 4, and 6), densities of total astrocytes (B) and total microglial cells (C) were significantly increased in the AD group, compared with the nondemented group. D: In the AD group, cortical thickness (righty axis and regression line with shaded error bars) correlated negatively with duration of clinical illness (r = −0.6538, P < 0.0001), indicating that disease duration is a reliable proxy of dementia severity. Unlike the total number of astrocytes (Figures 2 and 4), density (lefty axis) of all astrocytes (solid symbols) correlated positively with disease duration (r = 0.6400, P < 0.0001), because of progressive cortical atrophy; however, density of all microglia (open symbols) did not correlate significantly with disease duration (r = 0.0982, P = 0.5466). The 95% confidence interval (open error bars) of the mean slope is indicated. Data used to build the cortical thickness regression line for these 40 AD patients were drawn from Serrano-Pozo et al.∗P < 0.05, †P < 0.0001.
Similar articles
- Distinct changes in all major components of the neurovascular unit across different neuropathological stages of Alzheimer's disease.
Kirabali T, Rust R, Rigotti S, Siccoli A, Nitsch RM, Kulic L. Kirabali T, et al. Brain Pathol. 2020 Nov;30(6):1056-1070. doi: 10.1111/bpa.12895. Epub 2020 Sep 16. Brain Pathol. 2020. PMID: 32866303 Free PMC article. - Inflammatory pathology markers (activated microglia and reactive astrocytes) in early and late onset Alzheimer disease: a post mortem study.
Taipa R, Ferreira V, Brochado P, Robinson A, Reis I, Marques F, Mann DM, Melo-Pires M, Sousa N. Taipa R, et al. Neuropathol Appl Neurobiol. 2018 Apr;44(3):298-313. doi: 10.1111/nan.12445. Epub 2017 Nov 27. Neuropathol Appl Neurobiol. 2018. PMID: 29044639 - Microglia-Astrocyte Communication in Alzheimer's Disease.
Wu Y, Eisel ULM. Wu Y, et al. J Alzheimers Dis. 2023;95(3):785-803. doi: 10.3233/JAD-230199. J Alzheimers Dis. 2023. PMID: 37638434 Free PMC article. Review. - Microglia and Astrocytes in Alzheimer's Disease in the Context of the Aberrant Copper Homeostasis Hypothesis.
Pal A, Rani I, Pawar A, Picozza M, Rongioletti M, Squitti R. Pal A, et al. Biomolecules. 2021 Oct 28;11(11):1598. doi: 10.3390/biom11111598. Biomolecules. 2021. PMID: 34827595 Free PMC article. Review.
Cited by
- Gliovascular alterations in sporadic and familial Alzheimer's disease: APOE3 Christchurch homozygote glioprotection.
Henao-Restrepo J, López-Murillo C, Valderrama-Carmona P, Orozco-Santa N, Gomez J, Gutiérrez-Vargas J, Moraga R, Toledo J, Littau JL, Härtel S, Arboleda-Velásquez JF, Sepulveda-Falla D, Lopera F, Cardona-Gómez GP, Villegas A, Posada-Duque R. Henao-Restrepo J, et al. Brain Pathol. 2023 Mar;33(2):e13119. doi: 10.1111/bpa.13119. Epub 2022 Sep 21. Brain Pathol. 2023. PMID: 36130084 Free PMC article. - Roles of neuropathology-associated reactive astrocytes: a systematic review.
Lawrence JM, Schardien K, Wigdahl B, Nonnemacher MR. Lawrence JM, et al. Acta Neuropathol Commun. 2023 Mar 13;11(1):42. doi: 10.1186/s40478-023-01526-9. Acta Neuropathol Commun. 2023. PMID: 36915214 Free PMC article. - The Multifaceted Neurotoxicity of Astrocytes in Ageing and Age-Related Neurodegenerative Diseases: A Translational Perspective.
Bouvier DS, Fixemer S, Heurtaux T, Jeannelle F, Frauenknecht KBM, Mittelbronn M. Bouvier DS, et al. Front Physiol. 2022 Mar 17;13:814889. doi: 10.3389/fphys.2022.814889. eCollection 2022. Front Physiol. 2022. PMID: 35370777 Free PMC article. Review. - Astrocytic changes with aging and Alzheimer's disease-type pathology in chimpanzees.
Munger EL, Edler MK, Hopkins WD, Ely JJ, Erwin JM, Perl DP, Mufson EJ, Hof PR, Sherwood CC, Raghanti MA. Munger EL, et al. J Comp Neurol. 2019 May 1;527(7):1179-1195. doi: 10.1002/cne.24610. Epub 2019 Jan 7. J Comp Neurol. 2019. PMID: 30578640 Free PMC article. - Reduced cognitive function, increased blood-brain-barrier transport and inflammatory responses, and altered brain metabolites in LDLr -/-and C57BL/6 mice fed a western diet.
Rutkowsky JM, Lee LL, Puchowicz M, Golub MS, Befroy DE, Wilson DW, Anderson S, Cline G, Bini J, Borkowski K, Knotts TA, Rutledge JC; Mouse Metabolic Phenotyping Center Imaging Working Group. Rutkowsky JM, et al. PLoS One. 2018 Feb 14;13(2):e0191909. doi: 10.1371/journal.pone.0191909. eCollection 2018. PLoS One. 2018. PMID: 29444171 Free PMC article.
References
- Beach T.G., Walker R., McGeer E.G. Patterns of gliosis in Alzheimer's disease and aging cerebrum. Glia. 1989;2:420–436. - PubMed
- Itagaki S., McGeer P.L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–182. - PubMed
- Cahoy J.D., Emery B., Kaushal A., Foo L.C., Zamanian J.L., Christopherson K.S., Xing Y., Lubischer J.L., Krieg P.A., Krupenko S.A., Thompson W.J., Barres B.A. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. - PMC - PubMed
- Ulvestad E., Williams K., Mørk S., Antel J., Nyland H. Phenotypic differences between human monocytes/macrophages and microglial cells studied in situ and in vitro. J Neuropathol Exp Neurol. 1994;53:492–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50-AG05134/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- R01 AG008487/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- AG08487/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous