Optimum parameters for freeze-drying decellularized arterial scaffolds - PubMed (original) (raw)
Optimum parameters for freeze-drying decellularized arterial scaffolds
William S Sheridan et al. Tissue Eng Part C Methods. 2013 Dec.
Abstract
Decellularized arterial scaffolds have achieved success in advancing toward clinical use as vascular grafts. However, concerns remain regarding long-term preservation and sterilization of these scaffolds. Freeze drying offers a means of overcoming these concerns. In this study, we investigated the effects of various freeze-drying protocols on decellularized porcine carotid arteries and consequently, determined the optimum parameters to fabricate a stable, preserved scaffold with unaltered mechanical properties. Freeze drying by constant slow cooling to two final temperatures ((Tf), -10 °C and -40 °C) versus instant freezing was investigated by histological examination and mechanical testing. Slow cooling to Tf= -10 °C produced a stiffer and less distensible response than the non freeze-dried scaffolds and resulted in disruption to the collagen fibers. The mechanical response of Tf= -40 °C scaffolds demonstrated disruption to the elastin network, which was confirmed with histology. Snap freezing scaffolds in liquid nitrogen and freeze drying to Tf= -40 °C with a precooled shelf at -60 °C produced scaffolds with unaltered mechanical properties and a histology resembling non-freeze-dried scaffolds. The results of this study demonstrate the importance of optimizing the nucleation and ice crystal growth/size to ensure homogenous drying, preventing extracellular matrix disruption and subsequent inferior mechanical properties. This new manufacturing protocol creates the means for the preservation and sterilization of decellularized arterial scaffolds while simultaneously maintaining the mechanical properties of the tissue.
Figures
FIG. 1.
Freeze drying of decellularized vascular scaffolds. (A) Three freeze-drying protocols were investigated, Tf=−10°C, Tf=−40°C and snap frozen samples in a precooled −60°C chamber to Tf=−40°C. (B) Freeze-dried sample on mandrel ensures even drying. (C) Scaffold retains shape after removal from mandrel.
FIG. 2.
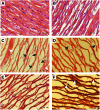
Effects of freeze drying on decellularized and customized scaffolds. (A) Highly cellular non-freeze-dried native tissue. (B) Cell removal reveals a porous intact ECM after decellularization. (C) Decreased matrix density is evident after customization. (D) Decellularized scaffolds in the Tf=−10°C group display a nonhomogenous matrix with fiber rupture within the wall center. (E) Tf=**−40°C shows markedly less fiber disruption than Tf=−10°C. (F) SF Tf=−40°C produced a homogenous structure with minimal ECM disruption for the decellularized scaffolds. (G) The Tf=−10°C customized scaffold showed evidence of fiber disruption but not as severe as the decellularized scaffold. (H) Fiber damage was visible for the customized scaffolds in the Tf=−40°C group with consistent drying. (I) SF Tf=−**40°C customized scaffolds resembled the non-freeze-dried scaffolds with homogenous drying. All scale bars indicate 200 μm. Color images available online at
FIG. 3.
ECM disruption as a result of freeze drying. (A) H&E staining of native tissue reveals highly dense undisrupted nature of collagen fibers. (B) Decellularization does not disrupt fibers (C) H&E staining shows that Tf=**−**10°C causes major collagen fiber disruption in decellularized scaffolds; black arrows indicate fully ruptured fibers; and white arrows display areas of nonuniform drying. (D) Less fiber damage was visible in Tf=−40°C decellularized scaffolds, but more uniform drying was apparent. (E) SF Tf=−40°C customized scaffolds retained a similar configuration of ECM as non-freeze-dried scaffolds. (F) Verhoff Van Gieson staining of decellularized scaffold in the Tf=−40°C group displayed disruption of the elastin network (elastin fibers stain black). Fragmentation of the elastin sheets was visible (white arrows). Scale bars in A–C indicate 20 μm and 50 μm in D. Color images available online at
FIG. 4.
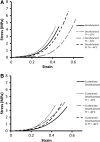
Stress**–**strain curves of freeze-dried scaffolds showing the mechanical response of the scaffolds (A) Decellularized scaffolds for Tf=−10°C produced a stiffer and less distensible response with an earlier than normal transition to the collagen region. Tf=−40°C displays a markedly different response with a large shift in the transition region of the graph extending the elastin dominant phase. SF Tf=−40°C matches closely the response of the non-freeze-dried scaffolds, particularly in the elastin dominant phase. (B) Customized scaffolds for Tf=−10°C produced a stiffer response with a much earlier transition region; similarly, for Tf=−40°C, an earlier transition region was seen with a less stiff collagen region. SF Tf=−40°C matched the response of the non-freeze-dried scaffolds for the elastin region, but an altered stiffer response was evident in the collagen phase.
FIG. 5.
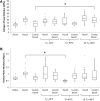
Collagen and elastin-phase moduli of native, decellularized, customized, and freeze-dried scaffolds. (A) Decellularization does not alter the collagen-phase modulus, while customization does. Freeze drying did not significantly affect the collagen modulus in any of the freeze-dried groups except the customized SF Tf=−40°C. (B) Decellularization increases the elastin-phase modulus, while customization reverses it toward the native modulus. Tf=**−**40°C significantly reduces the elastin-phase modulus for decellularized tissue, below both the native and decellularized moduli. However, SF Tf=−40°C maintains the elastin modulus for both decellularized and customized decellularized scaffolds. Statistical significance is indicated as *p<0.05.
FIG. 6.
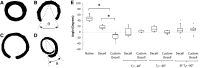
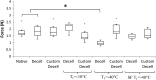
Opening angle measurement to determine residual stress within scaffolds. (A) Intact scaffold in stressed state. (B) Native scaffold in zero stress state achieved by radial cut of ring section producing an opening angle θ. (C) Decellularized scaffolds displayed a reduced value for θ compared with native scaffolds. (D) Customized scaffolds revealed an excessive reduction in θ to the extent that the scaffold recoiled beyond the diameter of the zero stress state. These θ values are taken as negative. (E) Boxplot of opening angles for each freeze-drying cycle. Decellularized scaffolds display a significant reduction in θ compared with the native tissue. Customized scaffolds see a significant decrease in θ due to collagen digestion, compared with both native tissue and decellularized scaffolds. Freeze drying reduces the opening angle for the decellularized scaffolds and increases this angle for each of the customized scaffolds, which can be attributed to collagen fiber damage and scaffold heterogeneity. The opening angles of the SF Tf=−40°C scaffolds are reverted back toward that of the native tissue. Statistical significance is indicated as *p<0.05.
FIG. 7.
Suture retention strength for native tissue and decellularized scaffolds. Decellularization, customization, and freeze drying had little effect on the suture retention strength. Tf=−40°C for decellularized scaffolds produced a significant drop in suture retention strength. Statistical significance is indicated as *p<0.05.
Similar articles
- Preservation strategies for decellularized pericardial scaffolds for off-the-shelf availability.
Zouhair S, Aguiari P, Iop L, Vásquez-Rivera A, Filippi A, Romanato F, Korossis S, Wolkers WF, Gerosa G. Zouhair S, et al. Acta Biomater. 2019 Jan 15;84:208-221. doi: 10.1016/j.actbio.2018.10.026. Epub 2018 Oct 17. Acta Biomater. 2019. PMID: 30342283 - Combination of freeze-thaw with detergents: A promising approach to the decellularization of porcine carotid arteries.
Cheng J, Wang C, Gu Y. Cheng J, et al. Biomed Mater Eng. 2019;30(2):191-205. doi: 10.3233/BME-191044. Biomed Mater Eng. 2019. PMID: 30741667 - Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying.
Grenier J, Duval H, Barou F, Lv P, David B, Letourneur D. Grenier J, et al. Acta Biomater. 2019 Aug;94:195-203. doi: 10.1016/j.actbio.2019.05.070. Epub 2019 May 30. Acta Biomater. 2019. PMID: 31154055 - Sucrose Diffusion in Decellularized Heart Valves for Freeze-Drying.
Wang S, Oldenhof H, Goecke T, Ramm R, Harder M, Haverich A, Hilfiker A, Wolkers WF. Wang S, et al. Tissue Eng Part C Methods. 2015 Sep;21(9):922-31. doi: 10.1089/ten.TEC.2014.0681. Epub 2015 May 4. Tissue Eng Part C Methods. 2015. PMID: 25809201 - Fundamentals of freeze-drying.
Nail SL, Jiang S, Chongprasert S, Knopp SA. Nail SL, et al. Pharm Biotechnol. 2002;14:281-360. doi: 10.1007/978-1-4615-0549-5_6. Pharm Biotechnol. 2002. PMID: 12189727 Review.
Cited by
- Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts.
Leal BBJ, Wakabayashi N, Oyama K, Kamiya H, Braghirolli DI, Pranke P. Leal BBJ, et al. Front Cardiovasc Med. 2021 Jan 11;7:592361. doi: 10.3389/fcvm.2020.592361. eCollection 2020. Front Cardiovasc Med. 2021. PMID: 33585576 Free PMC article. Review. - Enhancing organoid culture: harnessing the potential of decellularized extracellular matrix hydrogels for mimicking microenvironments.
Li C, An N, Song Q, Hu Y, Yin W, Wang Q, Le Y, Pan W, Yan X, Wang Y, Liu J. Li C, et al. J Biomed Sci. 2024 Sep 27;31(1):96. doi: 10.1186/s12929-024-01086-7. J Biomed Sci. 2024. PMID: 39334251 Free PMC article. Review. - Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering.
Carvalho MS, Silva JC, Udangawa RN, Cabral JMS, Ferreira FC, da Silva CL, Linhardt RJ, Vashishth D. Carvalho MS, et al. Mater Sci Eng C Mater Biol Appl. 2019 Jun;99:479-490. doi: 10.1016/j.msec.2019.01.127. Epub 2019 Jan 30. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30889723 Free PMC article. - Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects.
Golebiowska AA, Intravaia JT, Sathe VM, Kumbar SG, Nukavarapu SP. Golebiowska AA, et al. Bioact Mater. 2023 Oct 4;32:98-123. doi: 10.1016/j.bioactmat.2023.09.017. eCollection 2024 Feb. Bioact Mater. 2023. PMID: 37927899 Free PMC article. Review. - Photooxidation crosslinking to recover residual stress in decellularized blood vessel.
Wang J, Kong L, Gafur A, Peng X, Kristi N, Xu J, Ma X, Wang N, Humphry R, Durkan C, Zhang H, Ye Z, Wang G. Wang J, et al. Regen Biomater. 2021 Mar 13;8(2):rbaa058. doi: 10.1093/rb/rbaa058. eCollection 2021 Mar. Regen Biomater. 2021. PMID: 33738112 Free PMC article.
References
- Weinberg C. Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397. - PubMed
- Brar S. Stone G. Advances in percutaneous coronary intervention. Curr Cardiol Rep. 2009;11:245. - PubMed
- Macchiarini P. Jungebluth P. Go T. Asnaghi M.A. Rees L.E. Cogan T.A. Dodson A. Martorell J. Bellini S. Parnigotto P.P. Dickinson S.C. Hollander A.P. Mantero S. Conconi M.T. Birchall M.A. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023. - PubMed
- Burch P.T. Kaza A.K. Lambert L.M. Holubkov R. Shaddy R.E. Hawkins J. A. Clinical performance of decellularized cryopreserved valved allografts compared with standard allografts in the right ventricular outflow tract. Ann Thorac Surg. 2010;90:1301. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous