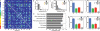Random convergence of olfactory inputs in the Drosophila mushroom body - PubMed (original) (raw)
Random convergence of olfactory inputs in the Drosophila mushroom body
Sophie J C Caron et al. Nature. 2013.
Abstract
The mushroom body in the fruitfly Drosophila melanogaster is an associative brain centre that translates odour representations into learned behavioural responses. Kenyon cells, the intrinsic neurons of the mushroom body, integrate input from olfactory glomeruli to encode odours as sparse distributed patterns of neural activity. We have developed anatomic tracing techniques to identify the glomerular origin of the inputs that converge onto 200 individual Kenyon cells. Here we show that each Kenyon cell integrates input from a different and apparently random combination of glomeruli. The glomerular inputs to individual Kenyon cells show no discernible organization with respect to their odour tuning, anatomic features or developmental origins. Moreover, different classes of Kenyon cells do not seem to preferentially integrate inputs from specific combinations of glomeruli. This organization of glomerular connections to the mushroom body could allow the fly to contextualize novel sensory experiences, a feature consistent with the role of this brain centre in mediating learned olfactory associations and behaviours.
Figures
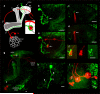
Figure 1. Dye-electroporation labels the PN connected to a KC claw
a Schematic illustration of the tracing strategy used to identify the PN connected to a single KC claw. PNs (one shown in red) transmit olfactory information from a single glomerulus in the AL to the MB by forming multiple axonal boutons in the calyx. KCs extend dendrites into the MB calyx (white) and project axons into either the α/β (light gray), α’/β' (medium gray), or γ (dark gray) lobe. The microglomerulus highlighted by a single photolabelled KC (green) is targeted for electroporation of red dye, resulting in the uptake of dye by a single PN and its associated AL glomerulus (red). Insert shows the targeted microglomerulus formed from a singe red PN bouton connected to the photolabelled KC claw (green) as well as other unlabeled KC claws (different shades of gray). b, Photolabelling of a single KC expressing PA-GFP under the control of the panneuronal promoter synaptobrevinGAL4 reveals 6 dendritic claws within the MB calyx. c, An electrode filled with Texas Red dextran is centered into the microglomerulus outlined by one of the photolabelled KC claws shown in b (arrow). d, Dye is electroporated into the targeted microglomerulus (arrow). e, Electroporated dye labels a single PN (n=684), which has a bouton that innervates the targeted microglomerulus (arrow). Note that the other KCs that synapse on this PN bouton were not labeled in this example. f, The photolabelled claw ensheathes the red dye-labeled PN bouton. Scale bar, 5 microns. g, The photolabelled KC projects to the α/β lobes of the MB whereas the dye-labeled PN it innervates the DM6 glomerulus h, A photolabelled KC with 3 claws. i, Three PNs innervating the DA1, VC4, and DL3 glomeruli are labeled upon loading all the claws of the KC depicted in **h.**Soma of the DA1 PN and VC4 PN are outlined while the DL3 soma is out of the plane. All scale bars are 10 microns except where noted.
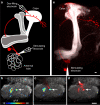
Figure 2. Dye-labeling identifies functional connections between PNs and KCs
a Schematic illustration of the strategy used to identify functional connections between PNs and KCs. An AL glomerulus (here DL3) is stimulated by local iontophoresis of acetylcholine (stimulating electrode). Optical recordings of calcium-mediated changes in fluorescence (ΔF/F) are measured in the MB calyx of a fly expressing GCaMP3 driven by the KC specific promoter OK107GAL4. A microglomerulus activated by the stimulation of DL3 is targeted for dye electroporation, identifying the pre-synaptic PN (red). b, Stimulation of the DL3 glomerulus activates several microglomeruli dispersed through the calyx. c, An electrode filled with Texas Red dextran is positioned into the center of an activated microglomerulus (arrow) highlighted by the recorded ΔF/F. d, Electroporation of dye into the targeted microglomerulus labels a single PN bouton (arrow). e, The labeled bouton extends from a single dye-filled PN that innervates the stimulated DL3 glomerulus (n=10). Note that the stimulating electrode is visualized by addition of Alexa-488 dextran dye to the acetylcholine. Scale bars are 10 microns.
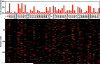
Figure 3. The connectivity matrix between AL glomeruli and KCs
The 665 connections between the AL glomeruli and KCs are represented in a matrix in the lower panel. Each row corresponds to one of the 200 photolabelled KCs while each column refers to the 51 AL glomeruli, the 2 thermo-sensing pseudoglomeruli and the other uncharacterized brain regions. Glomeruli connected once to a given KC are depicted as red bars. Glomeruli connected twice to the same KC are labeled as yellow bars. In the upper panel, the connections to all glomeruli and other brain regions are sorted according to their observed frequency.
Figure 4. KCs do not receive structured input
a Two glomeruli projecting to the same KC are considered a connected pair. All possible pairs of glomeruli are depicted as squares in a 53 x 53 matrix (51 AL glomeruli and 2 pseudoglomeruli), colored according to their observed frequency in the data (white outlined squares along the diagonal depict the frequency of identical pairs where a glomerulus is paired with itself). b, The frequency of KCs receiving two connections from the same glomerulus (an identical pair, gray bars) is compared to the frequency of such cells in 1,000 shuffled data sets (black circles: average, error bars: ± s.d.). c, The frequency of KCs receiving input from the same non-identical pair (gray bars) is compared to the frequency of such cells in 1,000 shuffled data sets (black circles: average, error bars: ± s.d.). d, Glomeruli are grouped based upon different anatomic or functional parameters,,-,. For each listed parameter, the percentage of connections across KCs receiving at least one input from a given group (as shown in f, g, and h for type of sensilla) is divided by the corresponding percentage observed in the full data set (as shown in e). A value of 1 for this quotient would indicate that the distributions across the selected KC groups and the full data set are identical. All analyses were also performed on 1,000 shuffled data sets (black circles, ± s.d.). e, The glomerular connections in the data set are grouped according to whether they receive input from an OSN that innervates a basiconic (blue), coeloconic (green), trichoid (red) or uncharacterized sensillum (gray). f-h, The distribution of the remaining glomerular connections to the 168 KCs receiving at least one input from a basiconic glomerulus, the 104 KCs receiving at least one input from a coeloconic glomerulus, and the 125 KCs receiving at least one input from a trichoid glomerulus are shown.
Comment in
- Neural circuits: random design of a higher-order olfactory projection.
Jacobson GA, Friedrich RW. Jacobson GA, et al. Curr Biol. 2013 May 20;23(10):R448-51. doi: 10.1016/j.cub.2013.04.016. Curr Biol. 2013. PMID: 23701688
Similar articles
- Presynaptic developmental plasticity allows robust sparse wiring of the Drosophila mushroom body.
Elkahlah NA, Rogow JA, Ahmed M, Clowney EJ. Elkahlah NA, et al. Elife. 2020 Jan 8;9:e52278. doi: 10.7554/eLife.52278. Elife. 2020. PMID: 31913123 Free PMC article. - A model of non-elemental olfactory learning in Drosophila.
Wessnitzer J, Young JM, Armstrong JD, Webb B. Wessnitzer J, et al. J Comput Neurosci. 2012 Apr;32(2):197-212. doi: 10.1007/s10827-011-0348-6. Epub 2011 Jun 23. J Comput Neurosci. 2012. PMID: 21698405 - Plasticity-driven individualization of olfactory coding in mushroom body output neurons.
Hige T, Aso Y, Rubin GM, Turner GC. Hige T, et al. Nature. 2015 Oct 8;526(7572):258-62. doi: 10.1038/nature15396. Epub 2015 Sep 30. Nature. 2015. PMID: 26416731 Free PMC article. - Mushroom body memoir: from maps to models.
Heisenberg M. Heisenberg M. Nat Rev Neurosci. 2003 Apr;4(4):266-75. doi: 10.1038/nrn1074. Nat Rev Neurosci. 2003. PMID: 12671643 Review. No abstract available. - Olfactory information processing in Drosophila.
Masse NY, Turner GC, Jefferis GS. Masse NY, et al. Curr Biol. 2009 Aug 25;19(16):R700-13. doi: 10.1016/j.cub.2009.06.026. Curr Biol. 2009. PMID: 19706282 Review.
Cited by
- Modeling and characterization of pure and odorant mixture processing in the Drosophila mushroom body calyx.
Lazar AA, Liu T, Yeh CH, Zhou Y. Lazar AA, et al. Front Physiol. 2024 Oct 16;15:1410946. doi: 10.3389/fphys.2024.1410946. eCollection 2024. Front Physiol. 2024. PMID: 39479309 Free PMC article. - The Neural Correlations of Olfactory Associative Reward Memories in Drosophila.
Lin YC, Wu T, Wu CL. Lin YC, et al. Cells. 2024 Oct 17;13(20):1716. doi: 10.3390/cells13201716. Cells. 2024. PMID: 39451234 Free PMC article. Review. - Whole-brain annotation and multi-connectome cell typing of Drosophila.
Schlegel P, Yin Y, Bates AS, Dorkenwald S, Eichler K, Brooks P, Han DS, Gkantia M, Dos Santos M, Munnelly EJ, Badalamente G, Serratosa Capdevila L, Sane VA, Fragniere AMC, Kiassat L, Pleijzier MW, Stürner T, Tamimi IFM, Dunne CR, Salgarella I, Javier A, Fang S, Perlman E, Kazimiers T, Jagannathan SR, Matsliah A, Sterling AR, Yu SC, McKellar CE; FlyWire Consortium; Costa M, Seung HS, Murthy M, Hartenstein V, Bock DD, Jefferis GSXE. Schlegel P, et al. Nature. 2024 Oct;634(8032):139-152. doi: 10.1038/s41586-024-07686-5. Epub 2024 Oct 2. Nature. 2024. PMID: 39358521 Free PMC article. - Reinforcement learning as a robotics-inspired framework for insect navigation: from spatial representations to neural implementation.
Lochner S, Honerkamp D, Valada A, Straw AD. Lochner S, et al. Front Comput Neurosci. 2024 Sep 9;18:1460006. doi: 10.3389/fncom.2024.1460006. eCollection 2024. Front Comput Neurosci. 2024. PMID: 39314666 Free PMC article. - The primacy model and the structure of olfactory space.
Giaffar H, Shuvaev S, Rinberg D, Koulakov AA. Giaffar H, et al. PLoS Comput Biol. 2024 Sep 10;20(9):e1012379. doi: 10.1371/journal.pcbi.1012379. eCollection 2024 Sep. PLoS Comput Biol. 2024. PMID: 39255274 Free PMC article.
References
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. - PubMed
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. - PubMed
- Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. - PubMed
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. - PubMed
- Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases