Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data - PubMed (original) (raw)
Review
Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data
Hansoo Kim et al. Br J Clin Pharmacol. 2014 Jan.
Abstract
In most therapeutic areas, multiple drug options are increasingly becoming available, but there is often a lack of evidence from head-to-head clinical trials that allows for direct comparison of the efficacy and/or safety of one drug vs. another. This review provides an introduction to, and overview of, common methods used for comparing drugs in the absence of head-to-head clinical trial evidence. Naïve direct comparisons are in most instances inappropriate and should only be used for exploratory purposes and when no other options are possible. Adjusted indirect comparisons are currently the most commonly accepted method and use links through one or more common comparators. Mixed treatment comparisons (MTCs) use Bayesian statistical models to incorporate all available data for a drug, even data that are not relevant to the comparator drug. MTCs reduce uncertainty but have not yet been widely accepted by researchers, nor drug regulatory and reimbursement authorities. All indirect analyses are based on the same underlying assumption as meta-analyses, namely that the study populations in the trials being compared are similar.
Keywords: adjusted indirect comparison; mixed treatment comparison; naïve direct comparison.
© 2013 The British Pharmacological Society.
Figures
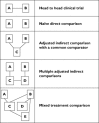
Figure 1
Conceptualization of the methods to compare the relative efficacies of drugs (or other interventions)
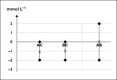
Figure 2
Illustration of uncertainty in a hypothetical adjusted indirect comparison. Two hypoglycaemic drugs, A and B, have been assessed with respect to reduction in blood glucose. Drug A was compared with drug C in a head-to-head clinical trial and drug B with drug C in another. Point estimates and uncertainty ranges of the differences in blood glucose change are illustrated. In the adjusted indirect comparison of A vs. B using C as a common comparator, the uncertainties in the pairwise comparisons of A vs. C and B vs. C are additive on the mmol l−1 scale
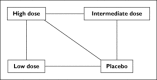
Figure 3
Conceptualization of a mixed treatment comparison undertaken by Ribeiro et al. [11], who investigated the impact of various statin doses (high, intermediate or low) and placebo on major cardiovascular events using evidence from 47 trials. Lines indicate the intervention groups that had been directly compared in the trials
Similar articles
- The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834 - Indirect comparisons of therapeutic interventions.
Schöttker B, Lühmann D, Boulkhemair D, Raspe H. Schöttker B, et al. GMS Health Technol Assess. 2009 Jul 21;5:Doc09. doi: 10.3205/hta000071. GMS Health Technol Assess. 2009. PMID: 21289896 Free PMC article. - Incorporating direct and indirect evidence using bayesian methods: an applied case study in ovarian cancer.
Griffin S, Bojke L, Main C, Palmer S. Griffin S, et al. Value Health. 2006 Mar-Apr;9(2):123-31. doi: 10.1111/j.1524-4733.2006.00090.x. Value Health. 2006. PMID: 16626416 - Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons.
Jansen JP, Crawford B, Bergman G, Stam W. Jansen JP, et al. Value Health. 2008 Sep-Oct;11(5):956-64. doi: 10.1111/j.1524-4733.2008.00347.x. Epub 2008 May 16. Value Health. 2008. PMID: 18489499 Review. - Ablation: Atrial fibrillation: diagnosis and management: Network meta-analysis J2.
National Guideline Centre (UK). National Guideline Centre (UK). London: National Institute for Health and Care Excellence (NICE); 2021 Apr. London: National Institute for Health and Care Excellence (NICE); 2021 Apr. PMID: 34165931 Free Books & Documents. Review.
Cited by
- Durability of glycaemic control with dapagliflozin, an SGLT2 inhibitor, compared with saxagliptin, a DPP4 inhibitor, in patients with inadequately controlled type 2 diabetes.
Bailey CJ, Del Prato S, Wei C, Reyner D, Saraiva G. Bailey CJ, et al. Diabetes Obes Metab. 2019 Nov;21(11):2564-2569. doi: 10.1111/dom.13841. Epub 2019 Aug 26. Diabetes Obes Metab. 2019. PMID: 31364269 Free PMC article. Clinical Trial. - The lack of head-to-head randomised trials and the consequences for patients and national health service: The case of non-small cell lung cancer.
Lasala R, Romagnoli A, Santoleri F, Isgrò V, Confalonieri C, Costantini A, Enrico F, Russo G, Polidori P, Di Paolo A, Malorgio F, Beretta G, Musicco F. Lasala R, et al. Eur J Clin Pharmacol. 2024 Apr;80(4):519-527. doi: 10.1007/s00228-024-03628-2. Epub 2024 Jan 20. Eur J Clin Pharmacol. 2024. PMID: 38244052 Clinical Trial. - The Value of Indirect Comparisons of Systemic Biologics for Psoriasis: Interpretation of Efficacy Findings.
Augustin M, Schuster C, Mert C, Nast A. Augustin M, et al. Dermatol Ther (Heidelb). 2022 Aug;12(8):1711-1727. doi: 10.1007/s13555-022-00765-3. Epub 2022 Jul 14. Dermatol Ther (Heidelb). 2022. PMID: 35834062 Free PMC article. Review. - Efficacy and safety of simple analgesics for acute treatment of episodic tension-type headache in adults: a network meta-analysis.
Xie R, Li J, Jing Y, Tian J, Li H, Cai Y, Wang Y, Chen W, Xu F. Xie R, et al. Ann Med. 2024 Dec;56(1):2357235. doi: 10.1080/07853890.2024.2357235. Epub 2024 May 30. Ann Med. 2024. PMID: 38813682 Free PMC article.
References
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. - PubMed
- PBAC. Canberra, Australia: 2008. Australian Department of Health and Ageing: Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (PBAC), Australian Department of Health and Ageing, Editor.
- NICE. Guide to the methods of technology appraisal, N.I.f.H.a.C. Excellence, Editor 2008.
- Well GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect Evidence: Indirect Treatment Comparisons in meta-Analysis, C.A.f.D.a.T.i. Health, Editor 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical