The cilium secretes bioactive ectosomes - PubMed (original) (raw)
The cilium secretes bioactive ectosomes
Christopher R Wood et al. Curr Biol. 2013.
Abstract
The release of membrane vesicles from the surface of cells into their surrounding environment is now recognized as an important pathway for the delivery of proteins to extracellular sites of biological function. Membrane vesicles of this kind, termed exosomes and ectosomes, are the result of active processes and have been shown to carry a wide array of biological effector molecules that can play roles in cell-to-cell communication and remodeling of the extracellular space. Degradation of the extracellular matrix (ECM) through the regulated release of proteolytic enzymes is a key process for development, morphogenesis, and cell migration in animal and plant cells. Here we show that the unicellular alga Chlamydomonas achieves the timely degradation of its mother cell wall, a type of ECM, through the budding of ectosomes directly from the membranes of its flagella. Using a combination of immunoelectron microscopy, immunofluorescence microscopy, and functional analysis, we demonstrate that these vesicles, which we term ciliary ectosomes, act as carriers of the proteolytic enzyme necessary for the liberation of daughter cells following mitosis. Chlamydomonas has proven to be the key unicellular model for the highly conserved mechanisms of mammalian cilia, and our results suggest that cilia may be an underappreciated source of bioactive, extracellular membrane vesicles.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
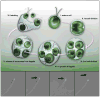
Figure 1. Illustration of the Chlamydomonas life-cycle
A cell grows to a threshold size (I), resorbs its flagella, then typically undergoes multiple rounds of cell division (II – III) within the original, mother cell wall. The resulting ball of cells is termed a sporangium. Daughter cells re-grow flagella while they are still inside of the sporangium (IV). Ectosomes carrying protease are released from the flagella of daughter cells (V). Hatching occurs when the ectosome-associated protease digests the mother cell wall liberating daughter cells from the sporangium (VI). The four lower panels display sequential frames taken from a DIC video micrograph of a flagellum of a vegetative Chlamydomonas cell. Black arrows indicate a ciliary ectosome budding from the tip of the flagellum. The source video, Movie S1, can be found in the supplemental data.
Figure 2. Ciliary ectosomes observed in the mature sporangium by electron microscopy
In the upper panel, an ultrathin section through a mature sporangium reveals five of the daughter cells within the mother cell wall prior to hatching. The location of the mother cell wall is emphasized by a dotted line. Four insets show higher magnification views of cross sections through flagella (black arrows indicate their position inside the sporangium). Numerous ectosomes are observed clustering around the flagella. The four lower panels display examples of ectosomes caught in the process of budding directly from the membranes of sporangial daughter cell flagella (white arrows). Inset scale bars indicate 100 nm.
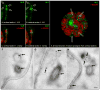
Figure 3. Localization of VLE protease on ciliary ectosomes in the mature sporangium
A and B are sequential, 250 nm, confocal sections through a flagellum of a daughter cell within a mature sporangium. VLE-specific fluorescence appears in green, and arrows point to discreet puncta clustered around the distal end of the flagellum. C and D display the same confocal sections as A and B, respectively, overlaying an α-tubulin-specific fluorescence channel in red (the flagellar axoneme). E shows a view from a 3D reconstruction of a mature sporangium consisting of 8 daughter cells preparing to hatch from their mother cell wall. VLE-specific fluorescence appears as green discreet puncta populating the interior space of the sporangium, between the daughter cells and the mother cell wall. Arrows point to the distal regions of four flagella surrounded by clusters of VLE puncta. Representative images from in situ immunogold labeling of mature sporangia with VLE antibodies are shown in the bottom panels. F – H are electron micrographs of ultrathin sections through three different sporangia at a stage just prior to hatching. Flagella (F) emanating from daughter cells within the mother cell wall (emphasized by dotted lines) have released ectosomes with VLE protease on their surface. Arrows point to the location of VLE-specific gold particles. A scale bar in panel H indicates 100 nm.
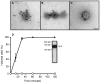
Figure 4. Isolated ciliary ectosomes carry VLE protease and induce hatching when added to hatching-defective mutants
A – C show numerous VLE-specific gold particles associated with the outer surfaces of isolated, intact ciliary ectosomes. The scale bar in C indicates 100 nm. D is a graph showing that ciliary ectosomes isolated from the medium of hatched pf1 cells induce complete hatching of ift88 mutants over a time course of ~30 minutes. Black circles indicate the data from ciliary ectosome addition. Open circles indicate control data from addition of the final supernatant from the ciliary ectosome preparation. Immunoblot analysis of isolated ectosomes with VLE antibody shows a band at ~125 kD.
Comment in
- Ciliary secretion: switching the cellular antenna to 'transmit'.
Avasthi P, Marshall W. Avasthi P, et al. Curr Biol. 2013 Jun 3;23(11):R471-3. doi: 10.1016/j.cub.2013.04.056. Curr Biol. 2013. PMID: 23743409
Similar articles
- Cilia-based peptidergic signaling.
Luxmi R, Kumar D, Mains RE, King SM, Eipper BA. Luxmi R, et al. PLoS Biol. 2019 Dec 6;17(12):e3000566. doi: 10.1371/journal.pbio.3000566. eCollection 2019 Dec. PLoS Biol. 2019. PMID: 31809498 Free PMC article. - Cilia-derived vesicles: An ancient route for intercellular communication.
Luxmi R, King SM. Luxmi R, et al. Semin Cell Dev Biol. 2022 Sep;129:82-92. doi: 10.1016/j.semcdb.2022.03.014. Epub 2022 Mar 26. Semin Cell Dev Biol. 2022. PMID: 35346578 Free PMC article. Review. - Current understandings of the relationship between extracellular vesicles and cilia.
Ikegami K, Ijaz F. Ikegami K, et al. J Biochem. 2021 Mar 5;169(2):139-145. doi: 10.1093/jb/mvaa112. J Biochem. 2021. PMID: 33035312 Review. - Regulated processing and secretion of a peptide precursor in cilia.
Luxmi R, Mains RE, Eipper BA, King SM. Luxmi R, et al. Proc Natl Acad Sci U S A. 2022 Aug 2;119(31):e2206098119. doi: 10.1073/pnas.2206098119. Epub 2022 Jul 25. Proc Natl Acad Sci U S A. 2022. PMID: 35878031 Free PMC article. - Comparative Analysis of Ciliary Membranes and Ectosomes.
Long H, Zhang F, Xu N, Liu G, Diener DR, Rosenbaum JL, Huang K. Long H, et al. Curr Biol. 2016 Dec 19;26(24):3327-3335. doi: 10.1016/j.cub.2016.09.055. Epub 2016 Nov 17. Curr Biol. 2016. PMID: 27866888 Free PMC article.
Cited by
- The Roles of Primary cilia in Polycystic Kidney Disease.
Kathem SH, Mohieldin AM, Nauli SM. Kathem SH, et al. AIMS Mol Sci. 2014;1(1):27-46. doi: 10.3934/molsci.2013.1.27. AIMS Mol Sci. 2014. PMID: 25599087 Free PMC article. - Mechanisms of Regulation in Intraflagellar Transport.
Mul W, Mitra A, Peterman EJG. Mul W, et al. Cells. 2022 Sep 2;11(17):2737. doi: 10.3390/cells11172737. Cells. 2022. PMID: 36078145 Free PMC article. Review. - Islet cilia and glucose homeostasis.
Melena I, Hughes JW. Melena I, et al. Front Cell Dev Biol. 2022 Dec 1;10:1082193. doi: 10.3389/fcell.2022.1082193. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36531945 Free PMC article. Review. - Single Gene Mutations in Pkd1 or Tsc2 Alter Extracellular Vesicle Production and Trafficking.
Kumar P, Zadjali F, Yao Y, Köttgen M, Hofherr A, Gross KW, Mehta D, Bissler JJ. Kumar P, et al. Biology (Basel). 2022 May 6;11(5):709. doi: 10.3390/biology11050709. Biology (Basel). 2022. PMID: 35625437 Free PMC article. - New software for automated cilia detection in cells (ACDC).
Lauring MC, Zhu T, Luo W, Wu W, Yu F, Toomre D. Lauring MC, et al. Cilia. 2019 Aug 1;8:1. doi: 10.1186/s13630-019-0061-z. eCollection 2019. Cilia. 2019. PMID: 31388414 Free PMC article.
References
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. Journal of proteomics. 2010;73:1907–1920. - PubMed
- Ginestra A, Monea S, Seghezzi G, Dolo V, Nagase H, Mignatti P, Vittorelli ML. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. J Biol Chem. 1997;272:17216–17222. - PubMed
- Dolo V, Ginestra A, Cassara D, Violini S, Lucania G, Torrisi MR, Nagase H, Canevari S, Pavan A, Vittorelli ML. Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res. 1998;58:4468–4474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources