A perspective on mammalian upstream open reading frame function - PubMed (original) (raw)
Review
A perspective on mammalian upstream open reading frame function
Joanna Somers et al. Int J Biochem Cell Biol. 2013 Aug.
Abstract
Post-transcriptional control makes a major contribution to the overall regulation of gene expression pathway. Within the cytoplasm this is mediated by a combination of regulatory RNA motifs within the 5' and 3' untranslated regions of mRNAs and their interacting protein/RNA partners. One of the most common regulatory RNA elements in mammalian transcripts (present in approximately 40% of all mRNAs) are upstream open reading frames (uORFs). However, despite the prevalence of these RNA elements how they function is not well understood. In general, they act to repress translation of the physiological ORF under control conditions, and under certain pathophysiological stresses this repression can be alleviated. It is known that re-initiation following the translation of an uORF is utilised in some situations however there are numerous alternative mechanisms that control the synthesis of a protein whose mRNA contains uORFs. Moreover, the trans-acting factors that are also involved in this process are not well defined. In this review we summarise our current understanding of this area and highlight some common features of these RNA motifs that have been discovered to date.
Keywords: 4E-binding proteins; 4EBPs; AdoMetDCS; EMCV; Eif; IRES; NMD; Protein synthesis; RRL; SNP; THPO; Translational control; UTR; Upstream open reading frame; adenosyl-methionine decarboxylase; encephalomyocarditis virus; eukaryotic initiation factor; internal ribosome entry segment; nonsense mediated decay; rabbit reticulocyte lysate; single nucleotide polymorphism; thrombopoietin; uORF; untranslated region; upstream open reading frame.
Copyright © 2013 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Fig. 1
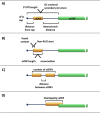
Translation initiation and its regulation. (A) Schematic of translation initiation pathway. Two complexes are required for initiation of translation; the eIF4F complex and the eIF2-TC. eIF2-TC interacts with the 40S ribosomal subunit to form the 43S preinitiation complex which then interacts (via eIF3 binding to eIF4G) with eIF4F complex to form the initiation competent 43S preinitiation complex. Translation initiation can be controlled by regulating the levels of eIF2-TC or eIF4F complex. (B) eIF4F complex is regulated by controlling the availability of the cap-binding protein eIF4E. Dephosphorylation of the eIF4E binding partners, 4EBPs, allows them to bind to eIF4E and so reduce the amount of this protein that is available for eIF4F complex formation. (C) eIF2-TC is controlled by phosphorylation of the alpha subunit of eIF2 by its kinases which results in sequestration of eIF2 and its GEF eIF2B in an inactive complex. This limits GTP:GDP recycling on eIF2 and therefore reduces the amount of eIF2-TC available.
Fig. 2
Many properties may contribute to an uORF's role in the translational control of a mORF. These include the length of the 5′UTR, the secondary structure and GC content. Consideration of where the uORF is situated, including the distance from the cap and the intercistronic distance between the termination of the uORF and the mORF (A). The sequence of the uORF might be important, whether it is an AUG or non-AUG initiator codon, the strength of the surrounding Kozak context and the uORF length and conservation. Conservation of uORFs may indicate a role for the peptide coding sequence of the uORF (B). Longer 5′UTRs tend to have multiple uORFs, so consideration of the distance between these uORFs is important (C). Lastly, some uORFs do not terminate within the 5′UTR, rather they overlap with the mORF. These uORFs if recognised by ribosomes will cause repression of the mORF (D).
Fig. 3
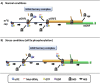
Reinitation mechanism of ATF4 mRNA translation. (A) Under normal conditions (abundant eIF2-TC), ribosomes that translate uORF1 (3 codons) may reinitate, that is the 40S remains attached to the transcript and resufmes scanning downstream. eIF2B acts as a GEF to eIF2-GDP, exchanging the GDP for GTP. Due to the abundant eIF2-TC availability the 40S ribosome reacquires a ternary complex before uORF2 allowing reinitation at uORF2 AUG codon. Hence, preventing translation of the ATF4 mORF. (B) Under stress conditions, which elevate eIF2α phosphorylation uORF1 in translated as described above. However, phosphorylated eIF2α on Ser 51 binds more strongly to eIF2B inhibiting its GEF function and reducing the available pool of eIF2-TC. This leads to the 40S ribosomes that resume scanning to progress further along the transcript before reacquiring eIF2-TC, thus bypassing the start codon of uORF2 to reinitiate at the ATF4 mORF.
Fig. 4
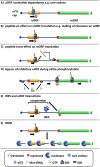
The roles of uORFs in translational control. In addition to reinitiation (Fig. 2) uORFs have been characterised to perform a number of different roles. (A) The nucleotide sequence can have a predominant role on uORF translatability, for instance by encoding for rare codons that cause the ribosome to stall (methionine synthase) or the potential involvement of such sequences in secondary structure or RNA motifs (UCP_2). The peptide product of uORFs can have cis regulatory functions, for instance causing the stalling of ribosomes (AdoMetDC and CHOP) (B), or by the trans repression of the mORF (AS, β_2 adrenergic receptor and vasopressin V1B receptor) (C). (D) Bypass of an inhibitory uORF has been observed under stress conditions and is dependent on eIF2α phosphorylation. An interesting possibility for how this may occur is the loading of the 40S without an eIF2-TC which it acquires as it scans (C/EBP α and β, CHOP, GADD34, protein kinase C isoform η). (E) Interactions that involve both uORFs and IRES elements within 5′UTRs can cause expression of the mORF (Cat-1) or repression of particular splice variants (VEGF-A). (F) Approximately 35–50% of uORF containing transcripts undergo selective degradation by NMD (e.g. ATF4, CHOP and IFDR1).
Similar articles
- Regulation of plant translation by upstream open reading frames.
von Arnim AG, Jia Q, Vaughn JN. von Arnim AG, et al. Plant Sci. 2014 Jan;214:1-12. doi: 10.1016/j.plantsci.2013.09.006. Epub 2013 Sep 18. Plant Sci. 2014. PMID: 24268158 Review. - Plant transcripts with long or structured upstream open reading frames in the NDL2 5' UTR can escape nonsense-mediated mRNA decay in a reinitiation-independent manner.
Cymerman MA, Saul H, Farhi R, Vexler K, Gottlieb D, Berezin I, Shaul O. Cymerman MA, et al. J Exp Bot. 2023 Jan 1;74(1):91-103. doi: 10.1093/jxb/erac385. J Exp Bot. 2023. PMID: 36169317 - Translation reinitiation after uORFs does not fully protect mRNAs from nonsense-mediated decay.
Russell PJ, Slivka JA, Boyle EP, Burghes AHM, Kearse MG. Russell PJ, et al. RNA. 2023 Jun;29(6):735-744. doi: 10.1261/rna.079525.122. Epub 2023 Mar 6. RNA. 2023. PMID: 36878710 Free PMC article. - Polyamine-responsive ribosomal arrest at the stop codon of an upstream open reading frame of the AdoMetDC1 gene triggers nonsense-mediated mRNA decay in Arabidopsis thaliana.
Uchiyama-Kadokura N, Murakami K, Takemoto M, Koyanagi N, Murota K, Naito S, Onouchi H. Uchiyama-Kadokura N, et al. Plant Cell Physiol. 2014 Sep;55(9):1556-67. doi: 10.1093/pcp/pcu086. Epub 2014 Jun 14. Plant Cell Physiol. 2014. PMID: 24929422 - Translational Regulation by Upstream Open Reading Frames and Human Diseases.
Silva J, Fernandes R, Romão L. Silva J, et al. Adv Exp Med Biol. 2019;1157:99-116. doi: 10.1007/978-3-030-19966-1_5. Adv Exp Med Biol. 2019. PMID: 31342439 Review.
Cited by
- Expression of human Hemojuvelin (HJV) is tightly regulated by two upstream open reading frames in HJV mRNA that respond to iron overload in hepatic cells.
Onofre C, Tomé F, Barbosa C, Silva AL, Romão L. Onofre C, et al. Mol Cell Biol. 2015 Apr;35(8):1376-89. doi: 10.1128/MCB.01462-14. Epub 2015 Feb 9. Mol Cell Biol. 2015. PMID: 25666510 Free PMC article. - No country for old methods: New tools for studying microproteins.
Valdivia-Francia F, Sendoel A. Valdivia-Francia F, et al. iScience. 2024 Jan 20;27(2):108972. doi: 10.1016/j.isci.2024.108972. eCollection 2024 Feb 16. iScience. 2024. PMID: 38333695 Free PMC article. Review. - Heme-regulated inhibitor: an overlooked eIF2α kinase in cancer investigations.
Yerlikaya A. Yerlikaya A. Med Oncol. 2022 May 15;39(5):73. doi: 10.1007/s12032-022-01668-1. Med Oncol. 2022. PMID: 35568791 Review. - Developmental regulation of canonical and small ORF translation from mRNAs.
Patraquim P, Mumtaz MAS, Pueyo JI, Aspden JL, Couso JP. Patraquim P, et al. Genome Biol. 2020 May 29;21(1):128. doi: 10.1186/s13059-020-02011-5. Genome Biol. 2020. PMID: 32471506 Free PMC article. - The Challenges and Strategies of Antisense Oligonucleotide Drug Delivery.
Gagliardi M, Ashizawa AT. Gagliardi M, et al. Biomedicines. 2021 Apr 16;9(4):433. doi: 10.3390/biomedicines9040433. Biomedicines. 2021. PMID: 33923688 Free PMC article. Review.
References
- Akimoto C., Sakashita E., Kasashima K., Kuroiwa K., Tominaga K., Hamamoto T. Translational repression of the McKusick-Kaufman syndrome transcript by unique upstream open reading frames encoding mitochondrial proteins with alternative polyadenylation sites. Biochimica et Biophysica Acta. 2012 epub pii: S0304-4165(12)00356-X. - PubMed
- Blagden S.P., Willis A.E. The biological and therapeutic relevance of mRNA translation in cancer. Nature Reviews Clinical Oncology. 2011;8:280–291. - PubMed
- Brown C.Y., Mize G.J., Pineda M., George D.L., Morris D.R. Role of two upstream open reading frames in the translational control of oncogene mdm2. Oncogene. 1999;18:5631–5637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_UP_A600_1023/MRC_/Medical Research Council/United Kingdom
- CRUK_/Cancer Research UK/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources