Deficiency in IL-17-committed Vγ4(+) γδ T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis - PubMed (original) (raw)
Deficiency in IL-17-committed Vγ4(+) γδ T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis
Elizabeth E Gray et al. Nat Immunol. 2013 Jun.
Abstract
Interleukin 17 (IL-17)-committed γδ T cells (γδT17 cells) participate in many immune responses, but their developmental requirements and subset specific functions remain poorly understood. Here we report that a commonly used CD45.1(+) congenic C57BL/6 mouse substrain is characterized by selective deficiency in Vγ4(+) γδT17 cells. This trait was due to a spontaneous mutation in the gene encoding the transcription factor Sox13 that caused an intrinsic defect in development of those cells in the neonatal thymus. The γδT17 cells migrated from skin to lymph nodes at low rates. In a model of psoriasis-like dermatitis, the Vγ4(+) γδT17 cell subset expanded considerably in lymph nodes and homed to inflamed skin. Sox13-mutant mice were protected from psoriasis-like skin changes, which identified a role for Sox13-dependent γδT17 cells in this inflammatory condition.
Figures
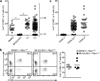
Figure 1. B6.SJL/NCI and B6.SJL/Tac mice lack Vγ4+ γδT17 cells
(a) Flow cytometric detection of Vγ4+CCR6+ γδ T cells in digested LN cell suspensions from B6/NCI and B6.SJL/NCI mice, gated on total γδ T cells. (b) Quantification of LN CCR6+ Vγ4+ and Vγ4− γδ T cell frequency (plotted as % of total γδ T cells) and absolute number in B6/NCI and B6.SJL/NCI mice gated as in (a). (c) Intracellular IL-17A staining of digested LN cell suspensions from B6/NCI and B6.SJL/NCI mice following PMA+I stimulation, gated on total γδ T cells. (d) Quantification of the absolute number of LN IL-17+ Vγ4+ and Vγ4− γδ T cells (left panel) and IL-17+ αβ T cells and TCR− cells (right panel) in B6/NCI and B6.SJL/NCI mice. (e) Quantification of the absolute number of SCART2+, SCART2−, and total CCR6+ γδ T cells in ear skin dermal cell suspensions from B6/NCI and B6.SJL/NCI mice. (f) Quantification of LN Vγ4+CCR6+ γδ T cell frequency in B6.SJL and SJL mice from various vendors gated as in (a), plotted as % of total γδ T cells. Each symbol represents an individual mouse; horizontal and vertical bars represent the mean (± s.d.) (b,d–f). *_P_≤0.01, **_P_≤0.005, ***_P_≤0.001. Data are representative of three experiments with 6–7 mice (a,b), three experiments with 4–6 mice (c,d), five experiments with 9–11 mice (e), and at least two experiments with 4 mice for each strain (f).
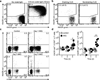
Figure 2. Vγ4+ γδT17 cell deficiency is an autosomal, recessive trait controlled by a single locus
(a) Quantification of LN Vγ4+CCR6+ γδ T cell frequency (plotted as % of total γδ T cells) in mice of the indicated type. (b) Flow cytometric detection of Vγ4+CCR6+ γδ T cells in digested lymph node cell suspensions from B6/NCI and B6.SJL/NCI × _Ptprc_−/− mice (3 to 6 weeks of age), gated on total γδ T cells (left panel); quantification of LN Vγ4+CCR6+ γδ T cell frequency (plotted as % of total γδ T cells) in mice of the indicated type (right panel). (c) Quantification of LN Vγ4+CCR6+ γδ T cell frequency (plotted as % of total γδ T cells) in F2 mice that are CD45.1+, CD45.1+/2+, and CD45.2+. The three recombination events are indicated by the filled squares. Each symbol represents an individual mouse; horizontal bars represent the mean (a–c). *_P_≤0.0001. Data are representative of 18 experiments with at least 15 mice of each type (a,c) and two experiments with 6 mice (b).
Figure 3. B6.SJL/NCI and B6.SJL/Tac mice harbor a frameshift mutation in Sox13
(a) Schematic of the Sox13 gene; exons are indicated in squares and introns by lines (not drawn to scale). The C nucleotide insertion and premature stop codon are indicated with arrows. (b) Sox13 cDNA and amino acid sequence in wild-type (WT, B6/NCI) and _Sox13_mut/mut (B6.SJL/NCI) mice. The C nucleotide insertion is indicated with an arrow. (c) Quantification of Sox13 mRNA in sorted Vγ4+CD24+ and Vγ4−CD24+ γδ T cells from adult thymi of WT (B6/NCI) and _Sox13_mut/mut (B6.SJL/NCI) mice, and whole thymus from a WT (B6/NCI) mouse. Real-time PCR data are shown for primer pairs spanning Sox13 exons 6–7 (left), exons 12–13 (center) and exons 1–2 (right). Horizontal and vertical bars represent the mean (± s.d.). *_P_≤0.05, **_P_≤0.01. Data are representative of two experiments (sorted γδ T cells) or one experiment (whole thymus).
Figure 4. Sox13 intrinsically regulates development of Vγ4+ γδT17 cells
(a) Flow cytometric detection of Vγ4+CCR6+ γδ T cells in digested LN cell suspensions from irradiated CD45.2+ _TCR_δ−/− mice reconstituted with BM from CD45.1+ WT (B6.SJL/Jax) or _Sox13_mut/mut (B6.SJL/NCI) mice and non-chimeric WT (B6/NCI) and _Sox13_mut/mut (B6.SJL/NCI) mice, gated on total γδ T cells. (b) Quantification of LN Vγ4+CCR6+ and Vγ4+CCR6− γδ T cell frequency (plotted as % of total γδ T cells, left panel) and dermal SCART2+CCR6+ γδ T cells (plotted as % of CD45.1+ dermal cells, right panel) in CD45.2+ _TCR_δ−/− mice reconstituted with BM of the indicated type. (c) Quantification of CD45.1+ LN Vγ4+CCR6+ and Vγ4+CCR6− and dermal SCART2+CCR6+ γδ T cells (plotted as % of indicated γδ T cell subset expressing CD45.1) in CD45.2+ _TCR_δ−/− mice reconstituted with a mixture of BM from CD45.2+ WT (B6/NCI) and CD45.1+ WT (B6.SJL/Jax) or _Sox13_mut/mut (B6.SJL/NCI) mice. Each symbol represents an individual mouse; horizontal and vertical bars represent the mean (± s.d.). *_P_≤0.1, **_P_≤0.01. Data are representative of three experiments with 3–7 mice.
Figure 5. Vγ4+ γδT17 development is blocked in the neonatal thymus in _Sox13_mut/mut mice
F1 mice were backcrossed to _Sox13_mut/mut (B6.SJL/NCI) mice to generate _Sox13_mut/+ or _Sox13_mut/mut neonates. (a,c) Intracellular IL-17A staining of thymocytes from day 0 and 5 neonates of the indicated type following stimulation with PMA+I, gated on Vγ4+ (a) or Vγ4− (c) γδ T cells. (b,d) Quantification of Vγ4+ (b) or Vγ4− (d) IL-17A+ γδ T cells from day 0 and 5 neonatal thymi of the indicated type. Each symbol represents an individual mouse, horizontal and vertical bars represent the mean (± s.d.). *_P_≤0.01, **_P_≤0.001, ***_P_≤0.0001. Data are representative three experiments with at least 9 mice.
Figure 6. _Sox13_mut/mut (B6.SJL/NCI) mice are protected from psoriasis-like dermatitis
(a) Quantification of ear skin thickness, plotted as fraction increase relative to baseline (day 0), of WT (B6/NCI) and _Sox13_mut/mut (B6.SJL/NCI) mice treated with imiquimod or control cream daily for 5 days. Boxes represent the mean (± s.d.). (b) H&E staining of ear skin from WT and _Sox13_mut/mut mice treated per (a) for 5 days. (c) Quantification of Ly6G+CD11b+ neutrophils in ear skin cell suspensions from WT and _Sox13_mut/mut mice treated per (a) for 3 or 5 days. Each symbol represents an individual mouse; horizontal bars represent the mean (± s.d.). (d) RT-PCR quantification of ear skin mRNA from WT and _Sox13_mut/mut mice treated with control (−) or imiquimod cream for 3 or 5 days. Boxes represent the mean (± s.d.). (e) Intracellular IL-17A staining of ear skin cell suspensions from WT and _Sox13_mut/mut mice treated as in (a) for 3 days and digested in the presence of Brefeldin A, gated on total γδ T cells. Mean (± s.d.) is indicated. (f) Transwell assay of neutrophil migration to ear skin supernatants prepared from WT and _Sox13_mut/mut mice treated as in (a) for 3 days. Each symbol represents migration from an individual transwell, horizontal bars represent the mean (± s.d.). *_P_≤0.05, **_P_≤0.01. Data are representative of three experiments with 3–6 mice (a–d), two experiments with 2–5 mice (e), and four experiments with 9 mice (f).
Figure 7. γδT17 cells migrate from skin to draining lymph nodes at low rates
(a) Flow cytometric detection of KikGR-red+ and KikGR-green+ cells in ear skin cell suspensions harvested immediately after exposure to violet light (right) or untreated (left), gated on total live cells. (b) Flow cytometric detection of KikGR-red+ CCR6+ γδ T cells in draining (left) and non-draining (right) cervical LNs (CLN) harvested 24 hours after ear skin photoconversion, gated on total CCR6+ γδ T cells. The mean (± s.d.) %KikGR-red+ cells from four independent experiments are indicated (n=4 mice). (c) Flow cytometric detection of KikGR-red+ CCR6+ and CCR6− γδT cells in draining CLNs of control and imiquimod-treated ear skin harvested at the indicated day of imiquimod treatment and one day after photoconversion, gated on total γδ T cells. (d) Quantification of KikGR-red+ CCR6+ and CCR6− γδ T cells in draining CLNs of control (empty squares) and imiquimod-treated (filled squares) ear skin, treated as in (c). Each symbol represents an individual CLN; horizontal bars represent the mean (± s.d.). *_P_≤0.05, **_P_≤0.001. Data are representative three experiments with 3 mice (a), four experiments with 4 mice (b), and 6 experiments with 3–7 mice at each time point (c,d).
Figure 8. Vγ4+ γδT17 cells expand in draining LNs and home to inflamed ear skin
(a) Intracellular IL-17A staining of PMA+I stimulated CLN cell suspensions from WT (B6/NCI) and _Sox13_mut/mut (B6.SJL/NCI) mice treated with imiquimod or control cream for 5 days, gated on total γδ T cells. (b,c) Quantification of IL-17A+ γδ T cells in CLNs (b) and blood (c) from mice treated as in (a). (d) Quantification Vγ4+Vδ4+ γδ T cell number in CLNs (left panel) or frequency in ear skin (right panel, plotted as % of live cells) treated as in (a) for 3–7 days. (e) Quantification of donor T cells in the indicated tissues, plotted as percent of CD45.2+ donor cells, from day 2 imiquimod-treated _Sox13_mut/mut recipients 3 hours after transfer of CLN cells from day 5 or 7 imiquimod-treated WT mice. (f) Quantification of donor T cells in the indicated tissues, plotted as percent of CD45.2+ (left panel) and IL-17A+CD45.2+ (right panel) donor cells from day 3 imiquimod-treated _Sox13_mut/mut recipients 2 days after transfer of CLN cells from day 5 imiquimod-treated WT mice. (g,h) Quantification of Ly6G+CD11b+ neutrophils, plotted as percent of CD45+ cells (g), and mRNA by RT-PCR (h) in ear skin from mice treated as in (f). Each symbol represents an individual mouse; horizontal bars represent the mean (± s.d.). *_P_≤0.05, **_P_≤0.01, ***_P_≤0.001. Data are representative of at least three experiments (a–e,g) or two experiments with at least 5 mice (f,h).
Similar articles
- IL-17-Producing Vγ4+ γδ T Cells Require Sphingosine 1-Phosphate Receptor 1 for Their Egress from the Lymph Nodes under Homeostatic and Inflammatory Conditions.
Maeda Y, Seki N, Kataoka H, Takemoto K, Utsumi H, Fukunari A, Sugahara K, Chiba K. Maeda Y, et al. J Immunol. 2015 Aug 15;195(4):1408-16. doi: 10.4049/jimmunol.1500599. Epub 2015 Jul 13. J Immunol. 2015. PMID: 26170380 - Development of interleukin-17-producing Vγ2+ γδ T cells is reduced by ICOS signaling in the thymus.
Buus TB, Schmidt JD, Bonefeld CM, Geisler C, Lauritsen JP. Buus TB, et al. Oncotarget. 2016 Apr 12;7(15):19341-54. doi: 10.18632/oncotarget.8464. Oncotarget. 2016. PMID: 27235509 Free PMC article. - IL-1β and IL-23 Promote Extrathymic Commitment of CD27+CD122- γδ T Cells to γδT17 Cells.
Muschaweckh A, Petermann F, Korn T. Muschaweckh A, et al. J Immunol. 2017 Oct 15;199(8):2668-2679. doi: 10.4049/jimmunol.1700287. Epub 2017 Aug 30. J Immunol. 2017. PMID: 28855314 Free PMC article. - Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses.
Ramírez-Valle F, Gray EE, Cyster JG. Ramírez-Valle F, et al. Proc Natl Acad Sci U S A. 2015 Jun 30;112(26):8046-51. doi: 10.1073/pnas.1508990112. Epub 2015 Jun 15. Proc Natl Acad Sci U S A. 2015. PMID: 26080440 Free PMC article. - Dissecting the complexity of γδ T-cell subsets in skin homeostasis, inflammation, and malignancy.
Castillo-González R, Cibrian D, Sánchez-Madrid F. Castillo-González R, et al. J Allergy Clin Immunol. 2021 Jun;147(6):2030-2042. doi: 10.1016/j.jaci.2020.11.023. Epub 2020 Nov 28. J Allergy Clin Immunol. 2021. PMID: 33259837 Review.
Cited by
- Recruitment of γδ T cells to the lesion via the CCL2/CCR2 signaling after spinal cord injury.
Xu P, Zhang F, Chang MM, Zhong C, Sun CH, Zhu HR, Yao JC, Li ZZ, Li ST, Zhang WC, Sun GD. Xu P, et al. J Neuroinflammation. 2021 Mar 2;18(1):64. doi: 10.1186/s12974-021-02115-0. J Neuroinflammation. 2021. PMID: 33653377 Free PMC article. - A Highly Focused Antigen Receptor Repertoire Characterizes γδ T Cells That are Poised to Make IL-17 Rapidly in Naive Animals.
Wei YL, Han A, Glanville J, Fang F, Zuniga LA, Lee JS, Cua DJ, Chien YH. Wei YL, et al. Front Immunol. 2015 Mar 23;6:118. doi: 10.3389/fimmu.2015.00118. eCollection 2015. Front Immunol. 2015. PMID: 25852688 Free PMC article. - The Jekyll and Hyde story of IL17-Producing γδT Cells.
Patil RS, Bhat SA, Dar AA, Chiplunkar SV. Patil RS, et al. Front Immunol. 2015 Feb 4;6:37. doi: 10.3389/fimmu.2015.00037. eCollection 2015. Front Immunol. 2015. PMID: 25699053 Free PMC article. Review. - γδ T cells in rheumatic diseases: from fundamental mechanisms to autoimmunity.
Nguyen CT, Maverakis E, Eberl M, Adamopoulos IE. Nguyen CT, et al. Semin Immunopathol. 2019 Sep;41(5):595-605. doi: 10.1007/s00281-019-00752-5. Epub 2019 Sep 10. Semin Immunopathol. 2019. PMID: 31506867 Free PMC article. Review. - The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells.
Heath WR, Carbone FR. Heath WR, et al. Nat Immunol. 2013 Oct;14(10):978-85. doi: 10.1038/ni.2680. Epub 2013 Sep 18. Nat Immunol. 2013. PMID: 24048119 Review.
References
- Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. - PubMed
- Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ gammadelta T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol. 2011;187:5026–5031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI045073/AI/NIAID NIH HHS/United States
- R01 AI045073/AI/NIAID NIH HHS/United States
- R37 AI045073/AI/NIAID NIH HHS/United States
- T32 AR007175/AR/NIAMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous