Targeting VEGF-VEGFR Pathway by Sunitinib in Peripheral Primitive Neuroectodermal Tumor, Paraganglioma and Epithelioid Hemangioendothelioma: Three Case Reports - PubMed (original) (raw)
Case Reports
Targeting VEGF-VEGFR Pathway by Sunitinib in Peripheral Primitive Neuroectodermal Tumor, Paraganglioma and Epithelioid Hemangioendothelioma: Three Case Reports
Tiziana Prochilo et al. Case Rep Oncol. 2013.
Abstract
Sunitinib malate (Sutent(TM); Pfizer Inc., New York, N.Y., USA) is a small molecule kinase inhibitor with activity against a number of tyrosine kinase receptors, including vascular endothelial growth factor receptors, stem-cell factor receptor, and platelet-derived growth factor receptors alpha and beta. Sunitinib, registered for the treatment of renal cell carcinoma and gastrointestinal stromal tumors, has recently been approved for the treatment of patients with advanced pancreatic neuroendocrine tumors. Peripheral primitive neuroectodermal tumor (pPNET), paraganglioma (PGL) and epithelioid hemangioendothelioma (EHE) are rare tumors in which there is an overexpression of pro-angiogenic factors and in which a high intratumoral microvessel density is a significant poor prognostic factor. On the basis of this preclinical rationale and the lack of effective treatments in pre-treated advanced stages of these rare diseases, we report our interesting experience of pPNET, PGL and EHE treatment with sunitinib.
Keywords: Antiangiogenic therapy; Epithelioid hemangioendothelioma; Paraganglioma; Peripheral primitive neuroectodermal tumor; Sunitinib.
Figures
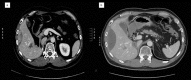
Fig. 1
a CT scan shows a solid lesion with compression of the right-side profile of VI and VIII hepatic segments attributable to recurrent disease. b After 2 courses of sunitinib treatment, a CT scan documents a change in the consistency of the lesion, which appear predominantly fluid.
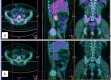
Fig. 2
a PET scan pretreatment showing the appearance of a new right iliac bone lesion. b PET scan after treatment documents response to sunitinib.
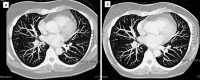
Fig. 3
a CT scan shows bilateral lung metastasis. b After 2 months of sunitinib treatment, a CT scan documents a partial response to treatment with the disappearance of some pulmonary nodules.
Similar articles
- Sunitinib malate.
Izzedine H, Buhaescu I, Rixe O, Deray G. Izzedine H, et al. Cancer Chemother Pharmacol. 2007 Aug;60(3):357-64. doi: 10.1007/s00280-006-0376-5. Epub 2006 Nov 30. Cancer Chemother Pharmacol. 2007. PMID: 17136543 - Inhibiting the VEGF-VEGFR pathway in angiosarcoma, epithelioid hemangioendothelioma, and hemangiopericytoma/solitary fibrous tumor.
Park MS, Ravi V, Araujo DM. Park MS, et al. Curr Opin Oncol. 2010 Jul;22(4):351-5. doi: 10.1097/CCO.0b013e32833aaad4. Curr Opin Oncol. 2010. PMID: 20485168 Review. - Metastatic hepatic epithelioid hemangio-endothelioma: long-term response to sunitinib malate.
Saada E, Saint Paul MC, Gugenheim J, Follana P, François E. Saada E, et al. Oncol Res Treat. 2014;37(3):124-6. doi: 10.1159/000360208. Epub 2014 Feb 21. Oncol Res Treat. 2014. PMID: 24685916 - Sunitinib: a newly approved small-molecule inhibitor of angiogenesis.
Cabebe E, Wakelee H. Cabebe E, et al. Drugs Today (Barc). 2006 Jun;42(6):387-98. doi: 10.1358/dot.2006.42.6.985633. Drugs Today (Barc). 2006. PMID: 16845442 Review. - Activity of sunitinib in patients with advanced neuroendocrine tumors.
Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X, Li JZ, Baum CM, Fuchs CS. Kulke MH, et al. J Clin Oncol. 2008 Jul 10;26(20):3403-10. doi: 10.1200/JCO.2007.15.9020. J Clin Oncol. 2008. PMID: 18612155 Clinical Trial.
Cited by
- Precision Oncology in Sarcomas: Divide and Conquer.
Carmagnani Pestana R, Groisberg R, Roszik J, Subbiah V. Carmagnani Pestana R, et al. JCO Precis Oncol. 2019 Apr 25;3:PO.18.00247. doi: 10.1200/PO.18.00247. eCollection 2019. JCO Precis Oncol. 2019. PMID: 32914012 Free PMC article. Review. - Therapies targeting the signal pathways of pheochromocytoma and paraganglioma.
Liu Y, Liu L, Zhu F. Liu Y, et al. Onco Targets Ther. 2019 Sep 4;12:7227-7241. doi: 10.2147/OTT.S219056. eCollection 2019. Onco Targets Ther. 2019. PMID: 31564906 Free PMC article. - Management and outcomes of advanced hemangioendothelioma at a medical oncology clinic in an Indian tertiary care center.
Tansir G, Rastogi S, Barwad A, Yadav R, Shamim SA, Dhamija E, Pandey R, Garg R, Shrivastava S. Tansir G, et al. Future Sci OA. 2023 Feb 3;8(10):FSO827. doi: 10.2144/fsoa-2021-0132. eCollection 2022 Dec. Future Sci OA. 2023. PMID: 36874373 Free PMC article. - Sunitinib in the therapy of malignant paragangliomas: report on the efficacy in a SDHB mutation carrier and review of the literature.
Canu L, Pradella S, Rapizzi E, Fucci R, Valeri A, Briganti V, Giachè V, Parenti G, Ercolino T, Mannelli M. Canu L, et al. Arch Endocrinol Metab. 2017 Jan-Feb;61(1):90-97. doi: 10.1590/2359-3997000000217. Epub 2016 Oct 10. Arch Endocrinol Metab. 2017. PMID: 27737332 Free PMC article. Review.
References
- De Bock K, Cauwenberghs S, Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev. 2011;21:73–79. - PubMed
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. - PubMed
- Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, et al. Food and Drug Administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12:107–113. - PubMed
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. - PubMed
- Leavey PJ, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, Children's Oncology Group et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2008;51:334–338. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources