Tumour necrosis factor alpha, interferon gamma and substance P are novel modulators of extrapituitary prolactin expression in human skin - PubMed (original) (raw)
Tumour necrosis factor alpha, interferon gamma and substance P are novel modulators of extrapituitary prolactin expression in human skin
Ewan A Langan et al. PLoS One. 2013.
Abstract
Human scalp skin and hair follicles (HFs) are extra-pituitary sources of prolactin (PRL). However, the intracutaneous regulation of PRL remains poorly understood. Therefore we investigated whether well-recognized regulators of pituitary PRL expression, which also impact on human skin physiology and pathology, regulate expression of PRL and its receptor (PRLR) in situ. This was studied in serum-free organ cultures of microdissected human scalp HFs and skin, i.e. excluding pituitary, neural and vascular inputs. Prolactin expression was confirmed at the gene and protein level in human truncal skin, where its expression significantly increased (p = 0.049) during organ culture. There was, however, no evidence of PRL secretion into the culture medium as measured by ELISA. PRL immunoreactivity (IR) in female human epidermis was decreased by substance P (p = 0.009), while neither the classical pituitary PRL inhibitor, dopamine, nor corticotropin-releasing hormone significantly modulated PRL IR in HFs or skin respectively. Interferon (IFN) γ increased PRL IR in the epithelium of human HFs (p = 0.044) while tumour necrosis factor (TNF) α decreased both PRL and PRLR IR. This study identifies substance P, TNFα and IFNγ as novel modulators of PRL and PRLR expression in human skin, and suggests that intracutaneous PRL expression is not under dopaminergic control. Given the importance of PRL in human hair growth regulation and its possible role in the pathogenesis of several common skin diseases, targeting intracutaneous PRL production via these newly identified regulatory pathways may point towards novel therapeutic options for inflammatory dermatoses.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
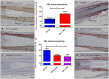
Figure 1. Prolactin immunoreactivity is present in corporal skin and its gene transcription can increase during culture.
Prolactin (PRL) immunoreactivity (IR) is demonstrated in (A) axillary skin from a 49 year old ♀, (B) axillary skin from a 28 year old ♀ and (C) thigh skin from a 57 year old ♀. The black arrows show that the IR was most prominent in the basal layer of the epidermis, in a cytoplasmic distribution, in non-scalp skin. (D) Omission of the primary antibody served as the negative control and (E) the hair follicle outer root sheath keratinocytes and sebaceous gland served as internal positive controls. (F) PRL gene expression was below the level of detectability at day 0, but became detectable by day 7 during serum free organ culture in breast skin (from a 42 year old ♀) and (G) abdominal skin (from 63 year old ♀). At the protein expression level, PRL IR also increased between (H) day 0 and (I) day 7. (J) High magnification of PRL IR. Quantitative measurement of PRL IR, using Image J software is shown in (K). Results pooled from 3 females aged 42–63 years. Increased protein expression correlated with increased gene expression in two subjects over the same time period. However, in one subject PRL gene expression was readily detectable at day 0, but decreased significantly during organ culture (data not shown). qRT-PCR results could not be normalized, combined and expressed as fold change from day 0, as no PRL gene expression was detected at day 0 in 2/3 subjects. All scale bars represent 50 µm.
Figure 2. Prolactin receptor immunoreactivity and gene expression is present in corporal skin and decreases during culture.
PRL receptor (PRLR) IR is present in the epidermis of corporal from three female subjects aged 28–63 years (A–C). This IR was present in the basal layer of the epidermis (black arrows) in a cytoplasmic distribution. (D) Omission of the primary antibody served as the negative control. (E) PRLR gene expression was detectable in corporal skin from three female subjects (results pooled and expressed as fold change), aged 42–63 years, at day 0 and decreased significantly during serum-free organ culture. PRLR protein expression also significantly decreased during organ culture (F–H). High magnification of PRLR IR shown in (I). Protein expression results were pooled from 3 females aged 42–63 years as in Fig 1(J). Representative photomicrographs of PRLR IR during organ culture are both taken from abdominal skin from 63 year old ♀. All scale bars represent 50 µm.
Figure 3. Substance P decreases PRL immunoreactivity in human epidermis.
Epidermal PRL IR (black arrows) was examined in full-thickness human skin organ culture. (A) PRL IR in the control group was significantly greater than that in (B) Substance P treated skin. PRL IR was measured using Image J software, showing that (C) epidermal PRL IR was significantly decreased by 100 nM Substance P treatment (p = 0.009). Results were pooled from ♀ subjects aged 45 and 46 years. Scale bars represent 50 µm.
Figure 4. IFNγ increases PRL immunoreactivity in the hair follicle, whilst TNFα decreases follicular PRL immunoreactivity.
(A) PRL IR in control hair follicle outer root sheath keratinocytes (black arrows), (B) after INFγ treatment and (C) negative control. (D) PRLR IR is significantly increased by INFγ treatment (p = 0.044). Results were pooled from 3 ♀ subjects (Aged 44–68), 14–16 HFs per group in total. In contrast, TNFα 5 ng/ml significantly decreased PRL IR (E). PRL IR is shown in (F) control hair follicles and those treated with (G) TNFα 0.5 ng/ml and (H) TNFα 5 ng/ml. Results were pooled from 3 ♀ subjects (Aged 49–68) 18–23 HFs per group in total. Scale bars represent 50 µm.
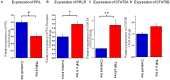
Figure 5. TNFα significantly decreases PRLR immunoreactivity in the outer root sheath of hair follicles.
(A) PRLR IR in the outer root sheath of control hair follicles (black arrows) was reduced in comparison PRLR IR after treatment with TNFα at concentrations of (B) 0.5 ng/ml and (C) 5 ng/ml. (D) Negative control. (E) Quantitative analysis confirms decreased PRLR IR after TNFα treatment (13–17 HFs per group in total). Results were pooled from same subjects described in Fig 4. (F) Pooled results of PRLR steady state gene expression in two subjects (♀ aged 53 and 66 years) showed no significant effect of 50 ng TNFα treatment after 48 hours. Scale bars represent 50 µm.
Figure 6. PRL and PRLR are detectable in cultured outer root sheath keratinocytes.
(A) PRL and PRLR were detectable in ORS keratinocytes in culture, consistent with the in-situ protein data. TNFα treatment (5 ng/ml) decreased PRL but (B) increased PRLR gene expression. There was also evidence that TNFα modulated STAT5a expression, a key downstream signal of PRLR, but not STAT5b (C–D).
Figure 7. STAT5 phosphorylation is increased by PRL treatment in serum-free skin organ culture.
Treatment with PRL (400 ng/ml) in serum-free organ culture increased epidermal STAT5 phosphorylation in all 3 of the individuals investigated. 3 females aged 42–63 years as in Fig 1(J) and 2(I). Arrows show phosphorylated STAT5 as detected by monoclonal rabbit antibody recognising STAT5a and b phosphorylated at Tyr 694.
Similar articles
- Thyrotropin-releasing hormone and oestrogen differentially regulate prolactin and prolactin receptor expression in female human skin and hair follicles in vitro.
Langan EA, Ramot Y, Hanning A, Poeggeler B, Bíró T, Gaspar E, Funk W, Griffiths CE, Paus R. Langan EA, et al. Br J Dermatol. 2010 May;162(5):1127-31. doi: 10.1111/j.1365-2133.2010.09676.x. Epub 2010 Mar 10. Br J Dermatol. 2010. PMID: 20302576 - Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine promoter of apoptosis-driven hair follicle regression.
Foitzik K, Krause K, Conrad F, Nakamura M, Funk W, Paus R. Foitzik K, et al. Am J Pathol. 2006 Mar;168(3):748-56. doi: 10.2353/ajpath.2006.050468. Am J Pathol. 2006. PMID: 16507890 Free PMC article. - Prolactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagen.
Foitzik K, Krause K, Nixon AJ, Ford CA, Ohnemus U, Pearson AJ, Paus R. Foitzik K, et al. Am J Pathol. 2003 May;162(5):1611-21. doi: 10.1016/S0002-9440(10)64295-2. Am J Pathol. 2003. PMID: 12707045 Free PMC article. - Prolactin: an emerging force along the cutaneous-endocrine axis.
Langan EA, Foitzik-Lau K, Goffin V, Ramot Y, Paus R. Langan EA, et al. Trends Endocrinol Metab. 2010 Sep;21(9):569-77. doi: 10.1016/j.tem.2010.06.001. Epub 2010 Jul 2. Trends Endocrinol Metab. 2010. PMID: 20598901 Review. - Prolactin (PRL) and prolactin receptor (PRLR) genes and their role in poultry production traits.
Wilkanowska A, Mazurowski A, Mroczkowski S, Kokoszyński D. Wilkanowska A, et al. Folia Biol (Krakow). 2014;62(1):1-8. doi: 10.3409/fb62_1.1. Folia Biol (Krakow). 2014. PMID: 24745142 Review.
Cited by
- Growth Hormone and the Human Hair Follicle.
Horesh EJ, Chéret J, Paus R. Horesh EJ, et al. Int J Mol Sci. 2021 Dec 8;22(24):13205. doi: 10.3390/ijms222413205. Int J Mol Sci. 2021. PMID: 34948002 Free PMC article. Review. - Changing human hair fibre colour and shape from the follicle.
Matamá T, Costa C, Fernandes B, Araújo R, Cruz CF, Tortosa F, Sheeba CJ, Becker JD, Gomes A, Cavaco-Paulo A. Matamá T, et al. J Adv Res. 2024 Oct;64:45-65. doi: 10.1016/j.jare.2023.11.013. Epub 2023 Nov 13. J Adv Res. 2024. PMID: 37967812 Free PMC article. - Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides.
Slominski AT, Slominski RM, Raman C, Chen JY, Athar M, Elmets C. Slominski AT, et al. Am J Physiol Cell Physiol. 2022 Dec 1;323(6):C1757-C1776. doi: 10.1152/ajpcell.00147.2022. Epub 2022 Nov 1. Am J Physiol Cell Physiol. 2022. PMID: 36317800 Free PMC article. Review. - Hormonal Effects on Hair Follicles.
Grymowicz M, Rudnicka E, Podfigurna A, Napierala P, Smolarczyk R, Smolarczyk K, Meczekalski B. Grymowicz M, et al. Int J Mol Sci. 2020 Jul 28;21(15):5342. doi: 10.3390/ijms21155342. Int J Mol Sci. 2020. PMID: 32731328 Free PMC article. Review. - Minireview: prolactin regulation of adult stem cells.
Sackmann-Sala L, Guidotti JE, Goffin V. Sackmann-Sala L, et al. Mol Endocrinol. 2015 May;29(5):667-81. doi: 10.1210/me.2015-1022. Epub 2015 Mar 20. Mol Endocrinol. 2015. PMID: 25793405 Free PMC article. Review.
References
- Dilme-Carreras E, Martin-Ezquerra G, Sanchez-Regana M, Umbert-Millet P (2011) Serum prolactin levels in psoriasis and correlation with cutaneous disease activity. Clin Exp Dermatol 36: 29–32. - PubMed
- Malligarjunan H, Gnanaraj P, Subramanian S, Elango T, Dayalan H (2011) Clinical efficacy of propylthiouracil and its influence on prolactin in psoriatic patients. Clin Biochem 44: 1209–1213. - PubMed
- El-Khateeb EA, Zuel-Fakkar NM, Eid SM, Abdul-Wahab SE (2011) Prolactin level is significantly elevated in lesional skin of patients with psoriasis. Int J Dermatol 50: 693–696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources