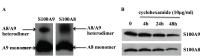Human S100A9 protein is stabilized by inflammatory stimuli via the formation of proteolytically-resistant homodimers - PubMed (original) (raw)
Human S100A9 protein is stabilized by inflammatory stimuli via the formation of proteolytically-resistant homodimers
Matteo Riva et al. PLoS One. 2013.
Abstract
S100A8 and S100A9 are Ca(2+)-binding proteins that are associated with acute and chronic inflammation and cancer. They form predominantly heterodimers even if there are data supporting homodimer formation. We investigated the stability of the heterodimer in myeloid and S100A8/S100A9 over-expressing COS cells. In both cases, S100A8 and S100A9 proteins were not completely degraded even 48 hrs after blocking protein synthesis. In contrast, in single transfected cells, S100A8 protein was completely degraded after 24 h, while S100A9 was completely unstable. However, S100A9 protein expression was rescued upon S100A8 co-expression or inhibition of proteasomal activity. Furthermore, S100A9, but not S100A8, could be stabilized by LPS, IL-1β and TNFα treatment. Interestingly, stimulation of S100A9-transfected COS cells with proteasomal inhibitor or IL-1β lead to the formation of protease resistant S100A9 homodimers. In summary, our data indicated that S100A9 protein is extremely unstable but can be rescued upon co-expression with S100A8 protein or inflammatory stimuli, via proteolytically resistant homodimer formation. The formation of S100A9 homodimers by this mechanism may constitute an amplification step during an inflammatory reaction.
Conflict of interest statement
Competing Interests: The authors have read the journal's policy and have the following conflicts: TL is a part time employee of Active Biotech AB. FI receives a research grant from Active Biotech AB. There are no further patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures
Figure 1. hS100A8 and hS100A9 form a stable heterodimer in THP-1.
THP-1 were treated with DSS for 30 min in ice. Subsequently samples were used for Western blot and stained for hS100A9 and hS100A8 (A). THP-1 cells were treated with 10 µg/ml cycloheximide for 4 h, 24 h and 48 h. Samples were, then, collected and Western blot for hS100A9 and hS100A8 was performed (B).
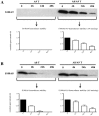
Figure 2. The hS100A8/hS100A9 heterodimers were more stable than hS100A8 and hS100A9 homodimers.
COS cells were transfected with hS100A8 and hS100A9 separately or together. After 24 h, COS cells were treated with 100 µg/ml cycloheximide for 4 h, 24 h and 48 h. Samples were collected and analyzed by Western blot. Filters were stained with (A) anti-hS100A9 or (B) anti-hS100A8. In the lower charts, the relative band intensity compared to non-stimulated cells are indicated.
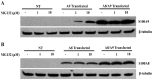
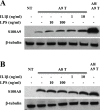
Figure 3. hS100A9 protein was unstable in COS cells but could be stabilized by MG132 or co-transfection with hS100A8.
COS cells were transfected with hS100A8 and hS100A9 constructs either separately or together. 24 h after transfection, the cells were stimulated for 8 h with 1 or 10 µM MG132 and subsequently analyzed by Western blot using (A) anti-hS100A9 or (B) anti-hS100A8. The first three lanes (NT) of each panel represented non-transfected cells. From lane 4 to 6, COS cells were transfected with (A; A9 Transfected) hS100A9 or (B; A8 Transfected) hS100A8 separately, while from lane 7 to 9 (A8/A9 Transfected) cells were co-transfected.
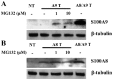
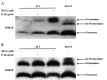
Figure 4. hS100A9 protein was unstable in LEP cells but could be rescued by MG132 and hS100A8.
hS100A8 and hS100A9 expression vectors were transfected into human fibroblasts (LEP cells) as described before for COS cells. After SDS-PAGE and Western blots were performed either with (A) anti-hS100A9 or (B) anti-hS100A8. Lane 1 (NT) represented non-transfected cells; from lane 2 to lane 4 cells were transfected with hS100A9 (panel A; A9 T) or with hS100A8 (panel B; A8 T); in lane 5 (A8/A9 T) cells were co-transfected with both constructs.
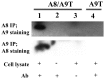
Figure 5. hS100A8 associates with hS100A9 in co-transfected COS cells.
COS cells were co-transfected with expression vectors for both hS100A8 and hS100A9. 24 h later, hS100A9 was immunoprecipitated and analyzed for hS100A8 expression by Western blot (Panel 2, lane 1). The same experiment was repeated immunoprecipitating hS100A8 and staining for hS100A9 (Panel 1, lane 1). Lane 2 and 3 represented the controls. In brief, in control samples, COS cells were co-transfected and a full Co-IP experiment was performed but without the cell extract, or the antibody, respectively. In panel 1, lane 4, COS cells were transfected only with hS100A9-carrying vector. The hS100A9 protein was immunoprecipitated and Western blot performed with hS100A8 staining.
Figure 6. LPS and IL1β induced hS100A9 protein stabilization.
COS cells were transfected either with hS100A8 or hS100A9 constructs, as described above. 24 h after transfection, COS cells were stimulated with either 1 or 10 ng/ml IL1β or alternatively with either 10 or 100 ng/ml LPS. Samples were collected and analyzed by Western blot. Filters were stained with (A) anti-hS100A9 and (B) anti-hS100A8. Lane 1 (NT) represented non-transfected cells; from lane 2 to 6, COS cells were transfected with (A; A9 T) hS100A9 or (B; A8 T) hS100A8 separately; in lane 7 (A8/A9 T) COS cells were co-transfected with both plasmids.
Figure 7. IL1β promotes the formation of protease-resistant hS100A9 homodimers.
COS cells were transfected with hS100A8 and hS100A9 expression vectors, either separately or together. 24 h after transfection, transfected cells were treated either with MG132 or IL1β. Then, COS cells were incubated with 1 mM DSS on ice for 30 minutes and analyzed by Western blot (hS100A9 (A) and hS100A8 (B)).
Similar articles
- S100A8/A9 mRNA induction in an ex vivo model of endotoxin tolerance: roles of IL-10 and IFNγ.
Fontaine M, Planel S, Peronnet E, Turrel-Davin F, Piriou V, Pachot A, Monneret G, Lepape A, Venet F. Fontaine M, et al. PLoS One. 2014 Jun 23;9(6):e100909. doi: 10.1371/journal.pone.0100909. eCollection 2014. PLoS One. 2014. PMID: 24956170 Free PMC article. - FGF-2, IL-1beta and TGF-beta regulate fibroblast expression of S100A8.
Rahimi F, Hsu K, Endoh Y, Geczy CL. Rahimi F, et al. FEBS J. 2005 Jun;272(11):2811-27. doi: 10.1111/j.1742-4658.2005.04703.x. FEBS J. 2005. PMID: 15943814 - The hetero-oligomeric complex of the S100A8/S100A9 protein is extremely protease resistant.
Nacken W, Kerkhoff C. Nacken W, et al. FEBS Lett. 2007 Oct 30;581(26):5127-30. doi: 10.1016/j.febslet.2007.09.060. Epub 2007 Oct 8. FEBS Lett. 2007. PMID: 17936757 - S100A8/A9 in Inflammation.
Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. Wang S, et al. Front Immunol. 2018 Jun 11;9:1298. doi: 10.3389/fimmu.2018.01298. eCollection 2018. Front Immunol. 2018. PMID: 29942307 Free PMC article. Review. - Inflammation-associated S100 proteins: new mechanisms that regulate function.
Goyette J, Geczy CL. Goyette J, et al. Amino Acids. 2011 Oct;41(4):821-42. doi: 10.1007/s00726-010-0528-0. Epub 2010 Mar 6. Amino Acids. 2011. PMID: 20213444 Review.
Cited by
- Calcium-induced Tetramerization and Zinc Chelation Shield Human Calprotectin from Degradation by Host and Bacterial Extracellular Proteases.
Stephan JR, Nolan EM. Stephan JR, et al. Chem Sci. 2016 Mar 1;7(3):1962-1975. doi: 10.1039/C5SC03287C. Epub 2015 Nov 23. Chem Sci. 2016. PMID: 26925211 Free PMC article. - Role of RAGE and Its Ligands on Inflammatory Responses to Brain Tumors.
Otazu GK, Dayyani M, Badie B. Otazu GK, et al. Front Cell Neurosci. 2021 Dec 16;15:770472. doi: 10.3389/fncel.2021.770472. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34975408 Free PMC article. Review. - Vesicular Location and Transport of S100A8 and S100A9 Proteins in Monocytoid Cells.
Chakraborty P, Bjork P, Källberg E, Olsson A, Riva M, Mörgelin M, Liberg D, Ivars F, Leanderson T. Chakraborty P, et al. PLoS One. 2015 Dec 14;10(12):e0145217. doi: 10.1371/journal.pone.0145217. eCollection 2015. PLoS One. 2015. PMID: 26661255 Free PMC article. - SERPINB3/B4 contributes to early inflammation and barrier dysfunction in an experimental murine model of atopic dermatitis.
Sivaprasad U, Kinker KG, Ericksen MB, Lindsey M, Gibson AM, Bass SA, Hershey NS, Deng J, Medvedovic M, Khurana Hershey GK. Sivaprasad U, et al. J Invest Dermatol. 2015 Jan;135(1):160-169. doi: 10.1038/jid.2014.353. Epub 2014 Aug 11. J Invest Dermatol. 2015. PMID: 25111616 Free PMC article. - Computational Deciphering of the Role of S100A8 and S100A9 Proteins and Their Changes in the Structure Assembly Influences Their Interaction with TLR4, RAGE, and CD36.
Paramasivam S, Perumal SS, Ekambaram SP. Paramasivam S, et al. Protein J. 2024 Apr;43(2):243-258. doi: 10.1007/s10930-024-10186-0. Epub 2024 Mar 2. Protein J. 2024. PMID: 38431537
References
- Schafer BW, Heizmann CW (1996) The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem. Sci 21: 134–140. - PubMed
- Marenholz I, Heizmann CW, Fritz G (2004) S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun 322: 1111–1122. - PubMed
- Salama I, Malone PS, Mihaimeed F, Jones JL (2008) A review of the S100 proteins in cancer. Eur J Surg Oncol 34: 357–364. - PubMed
- Foell D, Frosch M, Sorg C, Roth J (2004) Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta 344: 37–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous