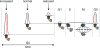Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis - PubMed (original) (raw)
Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis
Sander G Basten et al. Cilia. 2013.
Abstract
Dysfunctional cilia underlie a broad range of cellular and tissue phenotypes and can eventually result in the development of ciliopathies: pathologically diverse diseases that range from clinically mild to highly complex and severe multi-organ failure syndromes incompatible with neonatal life. Given that virtually all cells of the human body have the capacity to generate cilia, it is likely that clinical manifestations attributed to ciliary dysfunction will increase in the years to come. Disputed but nevertheless enigmatic is the notion that at least a subset of tumor phenotypes fit within the ciliopathy disease spectrum and that cilia loss may be required for tumor progression. Contending for the centrosome renders ciliation and cell division mutually exclusive; a regulated tipping of balance promotes either process. The mechanisms involved, however, are complex. If the hypothesis that tumorigenesis results from dysfunctional cilia is true, then why do the classic ciliopathies only show limited hyperplasia at best? Although disassembly of the cilium is a prerequisite for cell proliferation, it does not intrinsically drive tumorigenesis per se. Alternatively, we will explore the emerging evidence suggesting that some tumors depend on ciliary signaling. After reviewing the structure, genesis and signaling of cilia, the various ciliopathy syndromes and their genetics, we discuss the current debate of tumorigenesis as a ciliopathy spectrum defect, and describe recent advances in this fascinating field.
Figures
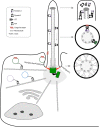
Figure 1
Structure of the primary cilium. The two centrioles are surrounded by the pericentrosomal matrix, serving as basal body and microtubule organizing center. Vesicular transport delivers ciliary components either to the basal body or ciliary pocket. Axonemal import is regulated by the transition zone, which is marked by Y-links. Anterograde kinesin-2- and retrograde dynein-2-mediated IFT sustain ciliary maintenance and cilia-dependent signaling; the complex rearranges at the ciliary tip.
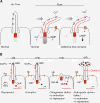
Figure 2
Ciliary signaling. (A) Mechanosensation. Flow induces cilia bending, the polycystin complex is a Ca2+ channel and causes an increase of intracellular Ca2+ levels that acts as a second messenger. In PKD, the polycystin complex fails to elevate intracellular Ca2+ hence mechanosensation is perturbed, leading to inappropriate responses and eventually cyst formation. (B) Schematic representation of Hh-signaling in normal conditions with and without Hh-ligand present. Abnormal Hh-signaling in the absence of primary cilia or in retrograde dynein-2 mutants.
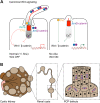
Figure 3
Canonical and non-canonical/PCP-signaling and cilia. (A) Canonical Wnt-signaling. When cilia are present, multiple mechanisms dampen Wnt-signaling; DVL is recruited by INVS/NPHP2 to the cilium, normal flow sensation elevates intracellular Ca2+ levels that switch Wnt-signaling off, Jouberin (Jbn) sequesters a pool of β-catenin and recruits it to the ciliary compartment. In cilia mutants, mislocalized INVS fails to recruit DVL, which translocates to the membrane and activates Wnt-signaling. Ca2+ response is lost, which fails to switch Wnt-signaling off. Jbn and β-catenin potentiate Wnt-signaling as a larger pool can translocate to the nucleus. (B) Illustrative model of cystic expansion of a renal tubule in a polycystic kidney. Disturbed PCP affects the orientation of cell division within the plane of tissue organization.
Figure 4
Cilia length in control of cell cycle progression. Cilia provide a physical block for cell cycle progression by laying claim to the basal body. Disassembly of the primary cilium is required to liberate the centrosome and allow duplication during S-phase and subsequent formation of the mitotic spindle during chromosomal segregation. Cilia mutants that inhibit ciliogenesis are prone to initiate rapid cell duplication when properly stimulated, and in contrast, increased axonemal length delays cell cycle progression. The maturation steps (growth and appendages) of the centrioles are indicated during the various cell cycle stages.
Figure 5
Model of cilia and cancer. We propose a number of pathways that can lead to cilia-dependent and cilia-independent tumor formation. The left pathway describes the oncogenic events that must follow after cilia have been lost (1) and a benign neoplasm has been formed; accumulation of genetic lesions (2) that desensitize cells to the microenvironment or CIN (3) to drive further cancer progression. Alternatively, the right pathway indicates a scenario where cilia are not involved in the initial transformation event (4). Depending on the underlying oncogenic mechanism, cells will further develop into a cilia-independent cancer (5), or will select for cilia retention in cilia-dependent cancer types (6).
Similar articles
- The perennial organelle: assembly and disassembly of the primary cilium.
Seeley ES, Nachury MV. Seeley ES, et al. J Cell Sci. 2010 Feb 15;123(Pt 4):511-8. doi: 10.1242/jcs.061093. J Cell Sci. 2010. PMID: 20144999 Free PMC article. Review. - Ciliary dysfunction in developmental abnormalities and diseases.
Sharma N, Berbari NF, Yoder BK. Sharma N, et al. Curr Top Dev Biol. 2008;85:371-427. doi: 10.1016/S0070-2153(08)00813-2. Curr Top Dev Biol. 2008. PMID: 19147012 Review. - Cilia kinases in skeletal development and homeostasis.
Abraham SP, Nita A, Krejci P, Bosakova M. Abraham SP, et al. Dev Dyn. 2022 Apr;251(4):577-608. doi: 10.1002/dvdy.426. Epub 2021 Oct 18. Dev Dyn. 2022. PMID: 34582081 Review. - Primary Cilia Reconsidered in the Context of Ciliopathies: Extraciliary and Ciliary Functions of Cilia Proteins Converge on a Polarity theme?
Hua K, Ferland RJ. Hua K, et al. Bioessays. 2018 Aug;40(8):e1700132. doi: 10.1002/bies.201700132. Epub 2018 Jun 8. Bioessays. 2018. PMID: 29882973 Free PMC article. Review. - The importance of a single primary cilium.
Mahjoub MR. Mahjoub MR. Organogenesis. 2013 Apr-Jun;9(2):61-9. doi: 10.4161/org.25144. Epub 2013 Apr 1. Organogenesis. 2013. PMID: 23819944 Free PMC article. Review.
Cited by
- Phosphoproteomic screening identifies physiological substrates of the CDKL5 kinase.
Muñoz IM, Morgan ME, Peltier J, Weiland F, Gregorczyk M, Brown FC, Macartney T, Toth R, Trost M, Rouse J. Muñoz IM, et al. EMBO J. 2018 Dec 14;37(24):e99559. doi: 10.15252/embj.201899559. Epub 2018 Sep 28. EMBO J. 2018. PMID: 30266825 Free PMC article. - Modulation of Primary Cilia by Alvocidib Inhibition of CILK1.
Wang EX, Turner JS, Brautigan DL, Fu Z. Wang EX, et al. Int J Mol Sci. 2022 Jul 23;23(15):8121. doi: 10.3390/ijms23158121. Int J Mol Sci. 2022. PMID: 35897693 Free PMC article. - Endothelial dysfunction in pulmonary arterial hypertension: loss of cilia length regulation upon cytokine stimulation.
Dummer A, Rol N, Szulcek R, Kurakula K, Pan X, Visser BI, Bogaard HJ, DeRuiter MC, Goumans MJ, Hierck BP. Dummer A, et al. Pulm Circ. 2018 Apr-Jun;8(2):2045894018764629. doi: 10.1177/2045894018764629. Epub 2018 Feb 26. Pulm Circ. 2018. PMID: 29480152 Free PMC article. - MKS1 regulates ciliary INPP5E levels in Joubert syndrome.
Slaats GG, Isabella CR, Kroes HY, Dempsey JC, Gremmels H, Monroe GR, Phelps IG, Duran KJ, Adkins J, Kumar SA, Knutzen DM, Knoers NV, Mendelsohn NJ, Neubauer D, Mastroyianni SD, Vogt J, Worgan L, Karp N, Bowdin S, Glass IA, Parisi MA, Otto EA, Johnson CA, Hildebrandt F, van Haaften G, Giles RH, Doherty D. Slaats GG, et al. J Med Genet. 2016 Jan;53(1):62-72. doi: 10.1136/jmedgenet-2015-103250. Epub 2015 Oct 21. J Med Genet. 2016. PMID: 26490104 Free PMC article. - Comparative sera proteomics analysis of differentially expressed proteins in oral squamous cell carcinoma.
Wong YL, Ramanathan A, Yuen KM, Mustafa WMW, Abraham MT, Tay KK, Rahman ZAA, Chen Y. Wong YL, et al. PeerJ. 2021 Jun 10;9:e11548. doi: 10.7717/peerj.11548. eCollection 2021. PeerJ. 2021. PMID: 34178453 Free PMC article.
References
- Stearns T. Centrosome Duplication: A Centriolar Pas de Deux. Cell. 2001;145:417–420. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases