Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing - PubMed (original) (raw)
Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing
Hua Liang et al. J Immunol. 2013.
Abstract
Local failures following radiation therapy are multifactorial, and the contributions of the tumor and the host are complex. Current models of tumor equilibrium suggest that a balance exists between cell birth and cell death due to insufficient angiogenesis, immune effects, or intrinsic cellular factors. We investigated whether host immune responses contribute to radiation-induced tumor equilibrium in animal models. We report an essential role for immune cells and their cytokines in suppressing tumor cell regrowth in two experimental animal model systems. Depletion of T cells or neutralization of IFN-γ reversed radiation-induced equilibrium, leading to tumor regrowth. We also demonstrate that PD-L1 blockade augments T cell responses, leading to rejection of tumors in radiation-induced equilibrium. We identify an active interplay between tumor cells and immune cells that occurs in radiation-induced tumor equilibrium and suggest a potential role for disruption of the PD-L1/PD-1 axis in increasing local tumor control.
Conflict of interest statement
Competing interests: The authors declare that no conflict of interest exists.
Figures
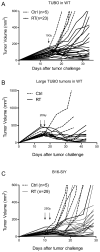
Figure 1
Ablative RT controls local tumor and induce stable/equilibrium diseases. (A) Balb/c wild type mice were s.c. inoculated with 5 ×105 TUBO. Tumors were irradiated with 15Gy 14 days later. (B) Larger TUBO tumors (200–400mm3) could be induced into equilibrium by higher dose of radiation. (C) B16-SIY tumors were allowed to grow for 10 days and then treated by two doses of 25Gy. Dotted lines: 0Gy control; solid lines: individual tumors received radiation. One of eight (A), two (B, C) representative experiments, respectively, is shown.
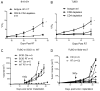
Figure 2
Host immune responses, not the radiosensitivity of cancer cells, correlate with efficacy of RT. (A) Tumor cells had the equal level of the sensitivity to radiation despite their differential early response to RT in vivo. Eight days after RT, non-RT control, non-responsive (NR) and responsive (R) tumors were removed and digested into cell suspension. The cells received 0Gy, 5Gy or 10Gy, and cultured for 7 days. Clonogenic assay was performed. (B) 21 days post RT, tumors were excised from partial response (PR), Stable (S) and Non-RT hosts and digested into cell suspension. The cells received 0Gy, 2Gy, 5Gy or 10Gy. Survival clonogenic assay was performed. The data was graphed as percentage compared to number of colonies in 0Gy control of each type of tumor. (C) IHC assay of the sequential slides of stable tumors stained with Ki-67, TUNEL and CD8 antibodies. Scale bar, 50μm. (D) Quantification of relative intensity of TUNEL staining in CD8+ and Ki67+ areas, 3–4 high power fields were counted per mouse. n=3. ** P=0.001. One of two (A, B) representative experiments is shown.
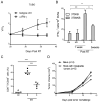
Figure 3
Antigen specific T cell responses are required for maintaining stable diseases. (A) Eighteen days after RT, CD8+ and CD4+ T cells were depleted in stable B16-SIY tumor. *p=0.016, n=8. V0 represents the tumor volume at the day of starting antibody treatment and V represents the tumor volume after antibody treatment. (B) Thirty five days after RT, mice with stable TUBO tumor were selected and depleted of CD8 or CD4 cells. *P=0.015, n=8. (C) Unlike in WT host, TUBO tumors in SCID mice could not reach equilibrium. Mice were treated as Fig. 1A. n=10. (D) TUBO tumors in BALB-neuT can be induced into equilibrium by RT. Tumors were irradiated with 30Gy 15 days later. Tumors in individual mouse were shown. One of two (A, C, D) or three (B) representative experiments is shown, respectively.
Figure 4
Antigen specific T cell responses are required for RT response as well as maintaining stable diseases. (A) Twenty five days after RT, mice bearing stable TUBO tumor were injected with IFNγ neutralizing antibody. *P=0.026, n=7. (B) Increased T cell response to neu antigen in DLNs in animals with responder tumors and stable tumors. DLNs were collected from control (non-RT group), non-responsive (NR), responsive group (R) 1 week after RT and stable group (S) 5 weeks after RT. IFNγ ELISPOT assays were performed. 3T3NKB cells were used for antigen presentation, 3T3KB as non-specific antigen control. *P<0.05, **P= 0.001, n=3/group. (C) CD8+ T cell frequency in both responder and stable tumors from (B) are elevated. ***P≤0.0005. (D) Systemic memory T cell response protects hosts from tumor re-challenge. Mice were re-challenged with 2×106 Tubo cells on the opposite flank 30 days after tumors were impalpable. One of three representative experiments is shown.
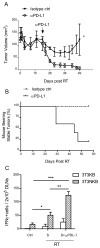
Figure 5
Blockade of PD-L1 breaks the equilibrium to favor tumor regression. Tumors were implanted and irradiated as Fig. 1A. Antibodies were administered 21 days after RT. (A) Tumors regressed after anti-PD-L1 injections. (B) Mice in treated group became tumor free over time. *P=0.018, n=5/group; (C) Antigen specific functional T cells were highly activated in stable tumors after neutralization of PD-L1. DLN of 4 mice per group were excised 5 weeks post RT (S) or 1 week post PD-L1 blockade (S+αPD-L1) and subjected to ELISPOT assays. *P=0.047; **P=0.002; ***P=0.0003. One of three representative experiments is shown.
Similar articles
- Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors.
Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Duraiswamy J, et al. Cancer Res. 2013 Jun 15;73(12):3591-603. doi: 10.1158/0008-5472.CAN-12-4100. Epub 2013 Apr 30. Cancer Res. 2013. PMID: 23633484 Free PMC article. - Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model.
Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, Allison JP, Waldmann TA. Yu P, et al. Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6187-92. doi: 10.1073/pnas.1203479109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474386 Free PMC article. - IFN-γ treatment protocol for MHC-Ilo/PD-L1+ pancreatic tumor cells selectively restores their TAP-mediated presentation competence and CD8 T-cell priming potential.
Stifter K, Krieger J, Ruths L, Gout J, Mulaw M, Lechel A, Kleger A, Seufferlein T, Wagner M, Schirmbeck R. Stifter K, et al. J Immunother Cancer. 2020 Aug;8(2):e000692. doi: 10.1136/jitc-2020-000692. J Immunother Cancer. 2020. PMID: 32868392 Free PMC article. - Disruption of SIRT7 Increases the Efficacy of Checkpoint Inhibitor via MEF2D Regulation of Programmed Cell Death 1 Ligand 1 in Hepatocellular Carcinoma Cells.
Xiang J, Zhang N, Sun H, Su L, Zhang C, Xu H, Feng J, Wang M, Chen J, Liu L, Shan J, Shen J, Yang Z, Wang G, Zhou H, Prieto J, Ávila MA, Liu C, Qian C. Xiang J, et al. Gastroenterology. 2020 Feb;158(3):664-678.e24. doi: 10.1053/j.gastro.2019.10.025. Epub 2019 Oct 31. Gastroenterology. 2020. PMID: 31678303 - What does PD-L1 positive or negative mean?
Ribas A, Hu-Lieskovan S. Ribas A, et al. J Exp Med. 2016 Dec 12;213(13):2835-2840. doi: 10.1084/jem.20161462. Epub 2016 Nov 30. J Exp Med. 2016. PMID: 27903604 Free PMC article. Review.
Cited by
- Non-canonical NF-κB Antagonizes STING Sensor-Mediated DNA Sensing in Radiotherapy.
Hou Y, Liang H, Rao E, Zheng W, Huang X, Deng L, Zhang Y, Yu X, Xu M, Mauceri H, Arina A, Weichselbaum RR, Fu YX. Hou Y, et al. Immunity. 2018 Sep 18;49(3):490-503.e4. doi: 10.1016/j.immuni.2018.07.008. Epub 2018 Aug 28. Immunity. 2018. PMID: 30170810 Free PMC article. - Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity.
Minniti G, Anzellini D, Reverberi C, Cappellini GCA, Marchetti L, Bianciardi F, Bozzao A, Osti M, Gentile PC, Esposito V. Minniti G, et al. J Immunother Cancer. 2019 Apr 11;7(1):102. doi: 10.1186/s40425-019-0588-y. J Immunother Cancer. 2019. PMID: 30975225 Free PMC article. - Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination.
Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R. Gong J, et al. J Immunother Cancer. 2018 Jun 4;6(1):46. doi: 10.1186/s40425-018-0361-7. J Immunother Cancer. 2018. PMID: 29866197 Free PMC article. Review. - Combining radiation and immunotherapy: a new systemic therapy for solid tumors?
Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, Massarelli E, Hong D, Naing A, Diab A, Gomez D, Ye H, Heymach J, Komaki R, Allison JP, Sharma P, Welsh JW. Tang C, et al. Cancer Immunol Res. 2014 Sep;2(9):831-8. doi: 10.1158/2326-6066.CIR-14-0069. Cancer Immunol Res. 2014. PMID: 25187273 Free PMC article. Review. - The Abscopal Effect in the Era of Checkpoint Inhibitors.
Kodet O, Němejcova K, Strnadová K, Havlínová A, Dundr P, Krajsová I, Štork J, Smetana K Jr, Lacina L. Kodet O, et al. Int J Mol Sci. 2021 Jul 4;22(13):7204. doi: 10.3390/ijms22137204. Int J Mol Sci. 2021. PMID: 34281259 Free PMC article. Review.
References
- Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev. 2011;21:42–49. - PubMed
- Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. - PMC - PubMed
- Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, Hug EB, Asbell SO, Grignon D. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. - PubMed
- Char DH, Kroll S, Phillips TL, Quivey JM. Late radiation failures after iodine 125 brachytherapy for uveal melanoma compared with charged-particle (proton or helium ion) therapy. Ophthalmology. 2002;109:1850–1854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA134563/CA/NCI NIH HHS/United States
- CA141975/CA/NCI NIH HHS/United States
- P01 CA097296/CA/NCI NIH HHS/United States
- CA111423/CA/NCI NIH HHS/United States
- R01 CA111423/CA/NCI NIH HHS/United States
- CA97296/CA/NCI NIH HHS/United States
- R01 CA141975/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials