The Arabidopsis NIMIN proteins affect NPR1 differentially - PubMed (original) (raw)
The Arabidopsis NIMIN proteins affect NPR1 differentially
Meike Hermann et al. Front Plant Sci. 2013.
Abstract
NON-EXPRESSOR OF PATHOGENESIS-RELATED GENES1 (NPR1) is the central regulator of the pathogen defense reaction systemic acquired resistance (SAR). NPR1 acts by sensing the SAR signal molecule salicylic acid (SA) to induce expression of PATHOGENESIS-RELATED (PR) genes. Mechanistically, NPR1 is the core of a transcription complex interacting with TGA transcription factors and NIM1-INTERACTING (NIMIN) proteins. Arabidopsis NIMIN1 has been shown to suppress NPR1 activity in transgenic plants. The Arabidopsis NIMIN family comprises four structurally related, yet distinct members. Here, we show that NIMIN1, NIMIN2, and NIMIN3 are expressed differentially, and that the encoded proteins affect expression of the SAR marker PR-1 differentially. NIMIN3 is expressed constitutively at a low level, but NIMIN2 and NIMIN1 are both responsive to SA. While NIMIN2 is an immediate early SA-induced and NPR1-independent gene, NIMIN1 is activated after NIMIN2, but clearly before PR-1. Notably, NIMIN1, like PR-1, depends on NPR1. In a transient assay system, NIMIN3 suppresses SA-induced PR-1 expression, albeit to a lesser extent than NIMIN1, whereas NIMIN2 does not negatively affect PR-1 gene activation. Furthermore, although binding to the same domain in the C-terminus, NIMIN1 and NIMIN2 interact differentially with NPR1, thus providing a molecular basis for their opposing effects on NPR1. Together, our data suggest that the Arabidopsis NIMIN proteins are regulators of the SAR response. We propose that NIMINs act in a strictly consecutive and SA-regulated manner on the SA sensor protein NPR1, enabling NPR1 to monitor progressing threat by pathogens and to promote appropriate defense gene activation at distinct stages of SAR. In this scenario, the defense gene PR-1 is repressed at the onset of SAR by SA-induced, yet instable NIMIN1.
Keywords: NIM1-INTERACTING (NIMIN) proteins; NON-EXPRESSOR OF PATHOGENESIS-RELATED GENES1 (NPR1); PATHOGENESIS-RELATED GENE1 (PR-1); plant defense gene activation; protein–protein interaction; salicylic acid (SA); systemic acquired resistance (SAR).
Figures
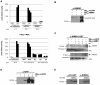
FIGURE 1
Arabidopsis NIMIN3 is expressed constitutively. (A) RT-PCR analyses of NIMIN3 expression in Arabidopsis whole seedlings and leaf tissue. Expression of NIMIN3 is compared to expression of NIMIN1, NIMIN2, and PR-1. RNA samples were isolated from 2-week-old whole seedlings grown either on MS medium or MS medium with addition of 0.3 mM SA and from leaves of 4-week-old plants 24 h after spraying with water or a suspension of Bion® containing 0.34 mM BTH. RT-PCR analyses were performed on DNase I-treated total RNA preparations in presence or absence of reverse transcriptase (RT) with primer combinations listed in Table 1. In lanes c, PCR products from 1 ng of plasmid DNAs carrying the respective cDNAs were loaded. The amplification of Actin1 mRNA serves as an internal standard for different RNA samples used in the amplification reactions. (B) Expression of a NIMIN3Pro::GUS reporter gene in transgenic tobacco seedlings. Expression from the NIMIN3 promoter is compared to reporter gene expression from the NIMIN1, NIMIN2, and Nt PR-1a promoters. Tobacco seedlings (T1 generation) transformed with the indicated reporter genes were grown on MS medium with kanamycin or on selective medium supplemented with 0.3 mM SA. Two independent lines for each construct or, as in case of the Nt PR-1a promoter, two different constructs were analyzed. Seedlings were stained for GUS reporter enzyme activity when 4-weeks-old.
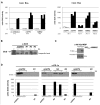
FIGURE 2
Salicylic acid-induced Arabidopsis NIMIN1 and NIMIN2 are expressed differentially from each other and from PR-1. RNA samples were isolated from Arabidopsis seedlings or Arabidopsis leaves and analyzed as described in Figure 1A. Expression of NIMIN1 and NIMIN2 is compared to expression of NIMIN3 and PR-1. (A) RT-PCR analyses of RNAs from wild-type (Col-0) and npr1-1 and npr1-2 mutant seedlings. 1-1, npr1-1; 1-2, npr1-2. (B) RT-PCR analyses of RNAs from leaf tissue at different times after spraying plants with 1 mM SA. (C) Time course of SA-induced GUS reporter enzyme activities and PR-1 protein accumulation in tobacco seedlings transformed with NIMIN1Pro::GUS or NIMIN2Pro::GUS. Expression from the two NIMIN promoters is compared to reporter gene expression from the Nt -1533PR-1a promoter. For immunodetection of endogenous PR-1 proteins, equal amounts of protein were loaded in each lane of the SDS gels. Seedlings (T1 generation) were grown on selective medium with 0.3 mM SA. Similar results were obtained with independent lines of NIMIN1Pro::GUS, NIMIN2Pro::GUS and -1533PR-1aPro::GUS.
FIGURE 3
Arabidopsis NIMIN1 and NIMIN3 suppress salicylic acid-induced gene expression from the tobacco PR-1a promoter in N. benthamiana. (A) Effects of transient expression of 35SPro::NIMIN1 and 35SPro::NIMIN3 in an N. benthamiana reporter line with integrated -1533PR-1aPro::GUS. Three plants were infiltrated in parallel for each gene construct with Agrobacterium strains as indicated. For a better direct comparison, the two halves of the same leaf were infiltrated with Agrobacteria harboring 35SPro::NIMIN1 and 35SPro::NIMIN3, respectively. Leaf disks excised from infiltrated leaf areas were floated on water or on 1 mM SA before determination of GUS enzyme activity. The three bars for each construct and treatment represent GUS activities from the three agroinfiltration experiments performed in parallel. Representative results are shown. N1, NIMIN1; N3, NIMIN3. (B) Immunodetection of NIMIN3 in extracts from agroinfiltrated and SA-floated leaf tissue. NIMIN3 accumulation was detected with a specific antiserum in an extract shown in Figure 3A. An unspecific band marked on the X-ray serves as loading control. Exposure of the X-ray film was for 1 min. (C) Immunodetection of NIMIN1 after agroinfiltration. Results from two independent time course experiments are shown. Leaf tissue was extracted after infiltration as indicated. Extracts were analyzed for protein accumulation with a specific antibody. As loading control, the region of the nitrocellulose filters with the small subunit of RuBisCO (SSU) stained with Ponceau S is shown. Exposure of the X-ray films was over night. dpi, days post-infiltration. (D) Immunodetection of green fluorescent protein (GFP) after agroinfiltration. Leaf tissue was extracted after infiltration as indicated. Exposure of the X-ray film was for 1 min. (E) Immunodetection of NIMIN1- and NIMIN3-Gal4 DNA binding domain (GBD) fusion proteins in extracts from transformed yeast. The NIMIN1 and NIMIN3 fusions were detected with the specific antisera used in Figures 3B,C.
FIGURE 4
Arabidopsis NIMIN2 does not affect salicylic acid-induced gene expression from the tobacco PR-1a promoter in N. benthamiana. Transient expression assays and immunodetection were performed as described in Figure 3. N1, NIMIN1; N2, NIMIN2; N3, NIMIN3. (A) Effects of transient expression of 35SPro::NIMIN2 in the N. benthamiana _-_1533PR-1aPro::GUS reporter line. The effects of NIMIN2 on the PR-1a::GUS reporter are compared to effects produced by NIMIN1 and NIMIN3. Representative results are shown. (B) Effects of transient expression of 35SPro::NIMIN1, 35SPro::NIMIN2, and 35SPro::NIMIN3 on accumulation of the GUS reporter protein in SA-treated leaf tissue. GUS accumulation was detected in extracts shown in Figure 4A. Lane c contains an extract from a tobacco plant stably transformed with 35SPro::GUS. An unspecific band marked on the X-ray serves as loading control. (C) Immunodetection of NIMIN2 in agroinfiltrated tissue. NIMIN2 accumulation was detected with a specific antiserum in an extract shown in Figure 4A. (D) Effects of transient expression of 35SPro::NIMIN1, 35SPro::NIMIN2, and 35SPro::NIMIN3 on accumulation of the endogenous PR-1 protein in SA-treated N. benthamiana leaf tissue. GUS reporter enzyme activities of extracts analyzed for PR-1 protein accumulation are given below the immunodetections.
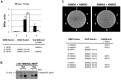
FIGURE 5
Arabidopsis NIMIN1, NIMIN2, and NIMIN3 do not bind simultaneously to At NPR1 in yeast. (A) Yeast two-hybrid interaction of At NPR1-Gal4 DNA binding domain (GBD) and NIMIN1-Gal4 activation domain (GAD) fusion proteins in absence and presence of NIMIN2. NIMIN2 was expressed from the Met25 promoter which is repressed in presence and de-repressed in absence of methionine. (B) Immunodetection of NIMIN2 in yeast. Yeast cells analyzed for lacZ reporter gene expression in Figure 5A were probed for accumulation of NIMIN2 protein. N2, NIMIN2. (C) Yeast three-hybrid interaction of At NPR1 with NIMIN1 and NIMIN3 or with NIMIN2 and NIMIN3. NIMIN1, NIMIN2, and NIMIN3 were expressed as fusions with the GBD or GAD. Simultaneous interaction of At NPR1 with GAD-TGA2 and GBD-NIMIN3 serves as positive control for formation of a ternary protein complex.
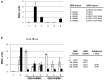
FIGURE 6
Arabidopsis NIMIN1 and NIMIN2 interact differentially with At NPR1 in yeast. (A) Yeast two-hybrid interaction of NIMIN1, NIMIN2 or NIMIN3 expressed as GBD fusions with the mutant protein At NPR1 F507/508S expressed as GAD fusion. Interactions of GAD-At NPR1 with GBD-NIMIN1 and GBD-NIMIN3 serve as positive controls. (B) Effect of SA on formation of ternary protein complexes comprising GBD-NIMIN1 or GBD-NIMIN2, GAD-At TGA2 or GAD-At TGA6 and At NPR1. The binary interactions of At NPR1 with NIMIN1 or NIMIN2 or TGA2 serve as controls for effects of SA (concentration 0.3 mM) on the NPR1–NIMIN1/2 interaction.
FIGURE 7
Working model for the consecutive action of Arabidopsis NIMIN proteins in the course of SAR. The model implies sequential interaction between diverse NIMIN proteins and NPR1 to form regulatory complexes with differential biochemical capacities in the course of SAR. The model also suggests that sensing of ambient SA levels in diseased plants may occur through the various NIMIN–NPR1 complexes, enabling activation of PR genes at distinct threshold levels of SA (indicated by steps). In this scenario, the defense gene PR-1 is induced late during SAR by direct action of SA on the NIMIN1–NPR1 regulatory complex.
Similar articles
- Tobacco NIMIN2 proteins control PR gene induction through transient repression early in systemic acquired resistance.
Zwicker S, Mast S, Stos V, Pfitzner AJ, Pfitzner UM. Zwicker S, et al. Mol Plant Pathol. 2007 Jul;8(4):385-400. doi: 10.1111/j.1364-3703.2007.00399.x. Mol Plant Pathol. 2007. PMID: 20507508 - Interaction of NIMIN1 with NPR1 modulates PR gene expression in Arabidopsis.
Weigel RR, Pfitzner UM, Gatz C. Weigel RR, et al. Plant Cell. 2005 Apr;17(4):1279-91. doi: 10.1105/tpc.104.027441. Epub 2005 Mar 4. Plant Cell. 2005. PMID: 15749762 Free PMC article. - NONEXPRESSOR OF PATHOGENESIS-RELATED PROTEINS1 (NPR1) and some NPR1-related proteins are sensitive to salicylic acid.
Maier F, Zwicker S, Hückelhoven A, Meissner M, Funk J, Pfitzner AJ, Pfitzner UM. Maier F, et al. Mol Plant Pathol. 2011 Jan;12(1):73-91. doi: 10.1111/j.1364-3703.2010.00653.x. Mol Plant Pathol. 2011. PMID: 21118350 Free PMC article. - Enhancement of broad-spectrum disease resistance in wheat through key genes involved in systemic acquired resistance.
Zhao S, Li M, Ren X, Wang C, Sun X, Sun M, Yu X, Wang X. Zhao S, et al. Front Plant Sci. 2024 Feb 23;15:1355178. doi: 10.3389/fpls.2024.1355178. eCollection 2024. Front Plant Sci. 2024. PMID: 38463563 Free PMC article. Review. - NPR1: the spider in the web of induced resistance signaling pathways.
Pieterse CM, Van Loon LC. Pieterse CM, et al. Curr Opin Plant Biol. 2004 Aug;7(4):456-64. doi: 10.1016/j.pbi.2004.05.006. Curr Opin Plant Biol. 2004. PMID: 15231270 Review.
Cited by
- Plant Protection against Viruses: An Integrated Review of Plant Immunity Agents.
Huang M, Wu Z, Li J, Ding Y, Chen S, Li X. Huang M, et al. Int J Mol Sci. 2023 Feb 23;24(5):4453. doi: 10.3390/ijms24054453. Int J Mol Sci. 2023. PMID: 36901884 Free PMC article. Review. - Transcription factor ANAC032 modulates JA/SA signalling in response to Pseudomonas syringae infection.
Allu AD, Brotman Y, Xue GP, Balazadeh S. Allu AD, et al. EMBO Rep. 2016 Nov;17(11):1578-1589. doi: 10.15252/embr.201642197. Epub 2016 Sep 15. EMBO Rep. 2016. PMID: 27632992 Free PMC article. - A Genetic Screen Identifies a Requirement for Cysteine-Rich-Receptor-Like Kinases in Rice NH1 (OsNPR1)-Mediated Immunity.
Chern M, Xu Q, Bart RS, Bai W, Ruan D, Sze-To WH, Canlas PE, Jain R, Chen X, Ronald PC. Chern M, et al. PLoS Genet. 2016 May 13;12(5):e1006049. doi: 10.1371/journal.pgen.1006049. eCollection 2016 May. PLoS Genet. 2016. PMID: 27176732 Free PMC article. - NPR1, a key immune regulator for plant survival under biotic and abiotic stresses.
Zavaliev R, Dong X. Zavaliev R, et al. Mol Cell. 2024 Jan 4;84(1):131-141. doi: 10.1016/j.molcel.2023.11.018. Epub 2023 Dec 15. Mol Cell. 2024. PMID: 38103555 Free PMC article. Review. - Transcriptome and metabolome analysis of stress tolerance to aluminium in Vitis quinquangularis.
Wang Q, Xu Y, Zhang M, Zhu F, Sun M, Lian X, Zhao G, Duan D. Wang Q, et al. Planta. 2021 Oct 23;254(5):105. doi: 10.1007/s00425-021-03759-1. Planta. 2021. PMID: 34687358
References
- Blanco F., Salinas P., Cecchini N. M., Jordana X., Van Hummelen P., Alvarez M. E., et al. (2009). Early genomic responses to salicylic acid in Arabidopsis. Plant Mol. Biol. 70 79–102 - PubMed
- Canet J. V., Dobón A., Roig A., Tornero P. (2010). Structure–function analysis of npr1 alleles in Arabidopsis reveals a role for its paralogs in the perception of salicylic acid. Plant Cell Environ. 33 1911–1922 - PubMed
- Cao H., Glazebrook J., Clarke J. D., Volko S., Dong X. (1997). The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88 57–63 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous