Reciprocal deletion and duplication at 2q23.1 indicates a role for MBD5 in autism spectrum disorder - PubMed (original) (raw)
doi: 10.1038/ejhg.2013.67. Epub 2013 May 1.
Jill A Rosenfeld 2, Carmen Orellana 3, Bregje W M van Bon 4, Sara Halbach 5, Elena A Repnikova 6, Lauren Brick 7, Chumei Li 7, Lucie Dupuis 8, Monica Rosello 3, Swaroop Aradhya 9, D James Stavropoulos 10, Kandamurugu Manickam 11, Elyse Mitchell 12, Jennelle C Hodge 12, Michael E Talkowski 13, James F Gusella 14, Kory Keller 15, Jonathan Zonana 15, Stuart Schwartz 16, Robert E Pyatt 6, Darrel J Waggoner 5, Lisa G Shaffer 17, Angela E Lin 18, Bert B A de Vries 4, Roberto Mendoza-Londono 8, Sarah H Elsea 19
Affiliations
- PMID: 23632792
- PMCID: PMC3865402
- DOI: 10.1038/ejhg.2013.67
Reciprocal deletion and duplication at 2q23.1 indicates a role for MBD5 in autism spectrum disorder
Sureni V Mullegama et al. Eur J Hum Genet. 2014 Jan.
Abstract
Copy number variations associated with abnormal gene dosage have an important role in the genetic etiology of many neurodevelopmental disorders, including intellectual disability (ID) and autism. We hypothesize that the chromosome 2q23.1 region encompassing MBD5 is a dosage-dependent region, wherein deletion or duplication results in altered gene dosage. We previously established the 2q23.1 microdeletion syndrome and report herein 23 individuals with 2q23.1 duplications, thus establishing a complementary duplication syndrome. The observed phenotype includes ID, language impairments, infantile hypotonia and gross motor delay, behavioral problems, autistic features, dysmorphic facial features (pinnae anomalies, arched eyebrows, prominent nose, small chin, thin upper lip), and minor digital anomalies (fifth finger clinodactyly and large broad first toe). The microduplication size varies among all cases and ranges from 68 kb to 53.7 Mb, encompassing a region that includes MBD5, an important factor in methylation patterning and epigenetic regulation. We previously reported that haploinsufficiency of MBD5 is the primary causal factor in 2q23.1 microdeletion syndrome and that mutations in MBD5 are associated with autism. In this study, we demonstrate that MBD5 is the only gene in common among all duplication cases and that overexpression of MBD5 is likely responsible for the core clinical features present in 2q23.1 microduplication syndrome. Phenotypic analyses suggest that 2q23.1 duplication results in a slightly less severe phenotype than the reciprocal deletion. The features associated with a deletion, mutation or duplication of MBD5 and the gene expression changes observed support MBD5 as a dosage-sensitive gene critical for normal development.
Figures
Figure 1
Delineation of the 2q23.1 critical region. Schematic representation of _MBD5_-containing duplications in this report and key variants previously reported in the literature. Duplications are in blue and green, while deletions are in pink. Three previously reported duplications that were cytogenetically defined; thus, no coordinates were given. Boxes represent the minimum size of the deletions/duplications and the horizontal lines extend through gaps in coverage to show the maximum deletion/duplication sizes. Green boxes represent individuals with MBD5 expression studies reported here. Genes within the region are represented by orange boxes, and the shaded region shows the location of MBD5. All individuals are unrelated, and all cases are de novo with the exception of GC19184 (cases a and b) and GC3057 (cases a and b), 3424_3 (cases a and b) which represent parent/child pairs.
Figure 2
Features present in individuals with 2q23.1 duplication syndrome. Four individuals with 2q23.1 duplication are shown, (a) SMS409, 7 years; (b) SMS397, 7 years; (c) SMS376, 15 years; (d) Chung 2 2012, 17 years and (e) SMS381, 6 years. While some dysmorphic craniofacial features are present (high arched eyebrows, thin upper lip, periorbital fullness, downslanting palpebral fissures, triangular face), no significant consistent craniofacial findings among individuals with 2q23.1 duplication are appreciated.
Figure 3
Hand and foot anomalies present in individuals with 2q23.1 microduplication syndrome. (a) Hands of SMS376. Note the fifth finger clinodactyly. (b) Foot of SMS409. Note the large, broad first toe.
Figure 4
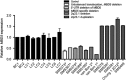
MBD5 expression is altered by deletion, duplication or disruption of the MBD5 gene. Quantitative real-time PCR expression analysis of MBD5 is shown in LCLs or peripheral blood lymphocytes from individuals with 2q23.1 deletions, 2q23.1 duplications, and normal controls using predesigned assays from Taqman MGB probes for MBD5 and GAPDH. GAPDH served as the endogenous control. Differences in transcript levels were quantified according to the ΔΔ_C_t method and normalized to GAPDH. Expression of all controls was normalized to 1.0. Acquired data were analyzed with 7500 Fast System SDS Software. Each bar represents mean (±SEM) of values from 3–10 independent experiments. Data show an expected range of expression for normal controls, 0.94- to 1.23-fold MBD5 expression in lymphocytes (BC1-2) and LCLs (LC1-7). Samples from 2q23.1 deletion syndrome patients show 0.22- to 0.59-fold expression of MBD5 (P<0.0001 for all cases). Samples from individuals with 2q23.1 duplication show overexpression of MBD5, ranging from 1.52- to 1.83-fold compared with normal controls (P<0.0001 for all cases). The expression changes observed indicate MBD5 is a dosage-sensitive gene. aTalkowski et al, 2011;bChung et al, 2012.
Similar articles
- Assessment of 2q23.1 microdeletion syndrome implicates MBD5 as a single causal locus of intellectual disability, epilepsy, and autism spectrum disorder.
Talkowski ME, Mullegama SV, Rosenfeld JA, van Bon BW, Shen Y, Repnikova EA, Gastier-Foster J, Thrush DL, Kathiresan S, Ruderfer DM, Chiang C, Hanscom C, Ernst C, Lindgren AM, Morton CC, An Y, Astbury C, Brueton LA, Lichtenbelt KD, Ades LC, Fichera M, Romano C, Innis JW, Williams CA, Bartholomew D, Van Allen MI, Parikh A, Zhang L, Wu BL, Pyatt RE, Schwartz S, Shaffer LG, de Vries BB, Gusella JF, Elsea SH. Talkowski ME, et al. Am J Hum Genet. 2011 Oct 7;89(4):551-63. doi: 10.1016/j.ajhg.2011.09.011. Am J Hum Genet. 2011. PMID: 21981781 Free PMC article. - 2q23.1 microdeletion of the MBD5 gene in a female with seizures, developmental delay and distinct dysmorphic features.
Noh GJ, Graham JM Jr. Noh GJ, et al. Eur J Med Genet. 2012 May;55(5):354-7. doi: 10.1016/j.ejmg.2012.05.003. Epub 2012 May 29. Eur J Med Genet. 2012. PMID: 22659271 - 2q23.1 microdeletion of the MBD5 gene in a female with seizures, developmental delay and distinct dysmorphic features.
Noh GJ, Graham JM Jr. Noh GJ, et al. Eur J Med Genet. 2012 Jan;55(1):59-62. doi: 10.1016/j.ejmg.2011.10.001. Epub 2011 Oct 24. Eur J Med Genet. 2012. PMID: 22085995 - Neurodevelopmental features in 2q23.1 microdeletion syndrome: report of a new patient with intractable seizures and review of literature.
Motobayashi M, Nishimura-Tadaki A, Inaba Y, Kosho T, Miyatake S, Niimi T, Nishimura T, Wakui K, Fukushima Y, Matsumoto N, Koike K. Motobayashi M, et al. Am J Med Genet A. 2012 Apr;158A(4):861-8. doi: 10.1002/ajmg.a.35235. Epub 2012 Mar 9. Am J Med Genet A. 2012. PMID: 22407754 Review. - Clinical and Molecular Aspects of MBD5-Associated Neurodevelopmental Disorder (MAND).
Mullegama SV, Elsea SH. Mullegama SV, et al. Eur J Hum Genet. 2016 Aug;24(9):1235-43. doi: 10.1038/ejhg.2016.35. Epub 2016 May 25. Eur J Hum Genet. 2016. PMID: 27222293 Free PMC article. Review.
Cited by
- Combining optical genome mapping and RNA-seq for structural variants detection and interpretation in unsolved neurodevelopmental disorders.
Xiao B, Luo X, Liu Y, Ye H, Liu H, Fan Y, Yu Y. Xiao B, et al. Genome Med. 2024 Sep 19;16(1):113. doi: 10.1186/s13073-024-01382-9. Genome Med. 2024. PMID: 39300495 Free PMC article. - Novel facial characteristics in congenital rubella syndrome: a study of 115 cases in a cardiac hospital of Bangladesh.
Begum NNF. Begum NNF. BMJ Paediatr Open. 2020 Nov 26;4(1):e000860. doi: 10.1136/bmjpo-2020-000860. eCollection 2020. BMJ Paediatr Open. 2020. PMID: 33305019 Free PMC article. - Germline mosaicism in a family with MBD5 haploinsufficiency.
Bhatia M, Cavalleri GL, White M, Delanty N, Sweeney BJ, Costello DJ, Greally MT, Benson KA. Bhatia M, et al. Cold Spring Harb Mol Case Stud. 2022 Dec 28;8(7):a006253. doi: 10.1101/mcs.a006253. Print 2022 Dec. Cold Spring Harb Mol Case Stud. 2022. PMID: 36396431 Free PMC article. - RINGs, DUBs and Abnormal Brain Growth-Histone H2A Ubiquitination in Brain Development and Disease.
Doyle LA, Unlu Bektas F, Chatzantonaki E, Repton C, Derrien A, Illingworth RS. Doyle LA, et al. Epigenomes. 2022 Dec 2;6(4):42. doi: 10.3390/epigenomes6040042. Epigenomes. 2022. PMID: 36547251 Free PMC article. Review. - The methyl binding domain containing protein MBD5 is a transcriptional regulator responsible for 2q23.1 deletion syndrome.
Walz K, Young JI. Walz K, et al. Rare Dis. 2014 Nov 3;2(1):e967151. doi: 10.4161/2167549X.2014.967151. eCollection 2014. Rare Dis. 2014. PMID: 26942102 Free PMC article.
References
- Matos A, Nogueira A, Criado B, Pereira S, Castedo S, Montenegro N. Prenatal diagnosis of partial trisomy 2q. Case report. Prenat Diagn. 1997;17:874–876. - PubMed
- Schumacher RE, Rocchini AP, Wilson GN. Partial trisomy 2q. Clin Genet. 1983;23:191–194. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous