Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta - PubMed (original) (raw)
. 2013 Jun;123(6):2408-20.
doi: 10.1172/JCI67039. Epub 2013 May 1.
Affiliations
- PMID: 23635772
- PMCID: PMC3668816
- DOI: 10.1172/JCI67039
Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta
Manyu Li et al. J Clin Invest. 2013 Jun.
Abstract
The remodeling of maternal uterine spiral arteries (SAs) is an essential process for ensuring low-resistance, high-capacitance blood flow to the growing fetus. Failure of SAs to remodel is causally associated with preeclampsia, a common and life-threatening complication of pregnancy that is harmful to both mother and fetus. Here, using both loss-of-function and gain-of-function genetic mouse models, we show that expression of the pregnancy-related peptide adrenomedullin (AM) by fetal trophoblast cells is necessary and sufficient to promote appropriate recruitment and activation of maternal uterine NK (uNK) cells to the placenta and ultimately facilitate remodeling of maternal SAs. Placentas that lacked either AM or its receptor exhibited reduced fetal vessel branching in the labyrinth, failed SA remodeling and reendothelialization, and markedly reduced numbers of maternal uNK cells. In contrast, overexpression of AM caused a reversal of these phenotypes with a concomitant increase in uNK cell content in vivo. Moreover, AM dose-dependently stimulated the secretion of numerous chemokines, cytokines, and MMPs from uNK cells, which in turn induced VSMC apoptosis. These data identify an essential function for fetal-derived factors in the maternal vascular adaptation to pregnancy and underscore the importance of exploring AM as a biomarker and therapeutic agent for preeclampsia.
Figures
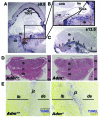
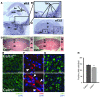
Figure 1. Fetal loss of Adm causes trophoblast apoptosis at the maternal-fetal interface.
(A) In situ hybridization of Adm gene expression in WT E9.5 mouse placentas, revealing robust expression in parietal TGCs. (B) Digital zoom of boxed region in A (enlarged ×2-fold), showing punctuate Adm staining in TGCs lining the ectoplacental cone (epc) at the innermost border of the maternal-fetal interface and little to no expression in the chorionic plate (cp), embryo (emb), or fetal membranes (fm). (C) Adm expression persisted in TGCs at E13.5 (arrows) and was diffusely expressed in stromal cells throughout the maternal decidua (de). The labyrinth (lb) and spongiotrophoblast-containing junctional zone (jz) expressed little to no_Adm_. (D) H&E staining of placentas from Adm+/+ and_Adm–/–_ littermates revealed largely normal structures, with no difference in the thickness of the labyrinth layer (Supplemental Figure 1). Digital zoom of central part of placentas is shown at the right of each image (enlarged ×1.5-fold). (E) TUNEL staining of placentas from_Adm+/+_ and_Adm–/–_ littermates showed a prominent band of apoptosing cells in_Adm–/–_ placentas that colocalized to TGC location and correlated with the high level of_Adm_ expression in these cells. For better clarity and data presentation, the original colors from the captured images in E were inverted using Adobe Photoshop. Original magnification, ×4 (A–C). Scale bars: 1 mm (D); 500 μM (E).
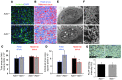
Figure 2. Reduced branching of fetal labyrinth vessels in Adm–/– placentas.
(A) Isolectin B4 staining revealed a highly branched network in_Adm+/+_ placentas, in contrast to abnormally large and underbranched fetal vessels in_Adm–/–_ placentas. (B) Fetal (blue) and maternal (red) blood sinuses demarcated within the labyrinth layer. (C) Quantitative morphometric analysis of total sinus space per field. (D) Quantitation of individual sinus area revealed that fetal sinuses of_Adm–/–_ placentas were significantly larger than those of Adm+/+ placentas, with a concomitant reduction in size of maternal sinuses. n = 12 placentas per genotype. *P < 0.02;#P < 0.01. (E andF) Scanning electron microscopy of vascular corrosion casts of maternal placental vasculature, at low power (E) and higher magnification (F). The large holes (white arrows) were indicative of large fetal vessels. Images are representative of n = 4 placentas per genotype. (G) Alkaline phosphatase staining of labyrinth revealed no structural or quantitative differences in chorionic villus cells. Data are mean ± SEM. Scale bars: 50 μM (A, B, and G); 1 mm (E); 100 μM (F).
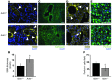
Figure 3. Fetal Adm dosage influences maternal SA remodeling and placental uNK cell content.
(A) Anti–α-SMA staining showed that decidual SAs of E13.5 Adm–/– placentas retained thick coverage of VSMCs (arrowheads) compared with those of_Adm+/+_ littermates. (B) Morphometric analysis showed a statistically significant increase in the thickness of SMA staining surrounding the SAs of_Adm–/–_ versus littermate_Adm+/+_ placentas. *P < 0.01. (C) Cathepsin staining of decidual SAs. (D) BrdU incorporation assays showed marked proliferation of vascular endothelial cells (arrowheads) in all SAs of Adm+/+ placentas, but this was rarely evident in SAs of_Adm–/–_ placentas. (E) Perforin staining of uNK cells within deciduas at E13.5. (F) Quantitation of perforin+ uNK cells showed a significant reduction in_Adm–/–_ versus_Adm+/+_ placentas. *P < 0.05. For all analyses, n = 10 placentas per genotype. Scale bars: 50 μM.
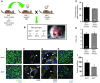
Figure 4. Ovary transplantation reveals independence of maternal genotype on Adm–/– placental phenotype.
(A) Ovary transplantation protocol. Donor ovaries from_Adm+/–_ or_Adm+/+_ female mice were surgically sutured in the ovarian bursa of WT 129S6/SvEv recipient mice after removal of their own ovaries. The recipient females were then bred to_Adm+/–_ male mice to generate_Adm+/+,Adm+/–, or_Adm–/– offspring. (B) Mendelian ratios of offspring were as expected, and_Adm–/–_ mice exhibited characteristic embryonic edema (arrowhead) at E13.5. (C andD) Recovery time to first parturition (C) and average litter size (D). Numbers within bars denote total number of litters analyzed per breeding. (E–I)Adm–/– placentas from_Adm+/–_ ovary→WT recipient females displayed the same pathological phenotypes of_Adm–/–_ placentas from_Adm+/–_ females — including (E) reduced fetal vessel branching in the labyrinth, (F) retention of SMCs (arrowheads) around maternal SAs, (G) reduction in endothelial cell proliferation (arrowheads) in maternal SAs, and (H and I) significantly reduced DBA+ uNK cell numbers — compared with_Adm+/+_ littermates. *P < 0.05. For ovary transplant studies, n = 6–10 placentas per genotype. Scale bars: 50 μM.
Figure 5. Fetal loss of Calcrl recapitulates Adm–/– placental phenotypes.
(A) In situ hybridization of Calcrl gene expression in WT E9.5 mouse placentas revealed expression in parietal TGCs, similar to the pattern of Adm expression. Unlike Adm,Calcrl was expressed at moderate levels in the embryo and fetal membranes. There was also robust expression of Calcrl in cord-like structures throughout the early decidua. (B) Digital zoom of boxed region in A (enlarged ×2-fold), showing punctuate Calcrl staining in TGCs lining the ectoplacental cone at the innermost border of the maternal-fetal interface and little to no expression in the chorionic plate. (C) Calcrl_expression was diffuse throughout the labyrinth at E13.5 and robustly expressed in the maternal endothelial cells lining the decidual SAs (arrows, inset; enlarged ×2-fold). (D) H&E staining of placentas from_Calcrl+/+ and_Calcrl–/–_ littermates revealed largely normal structures, with no appreciable differences in layers. Digital zoom of central part of placentas is shown at the right of each image (enlarged ×1.5-fold). (E–H)Calcrl–/– placentas at E13.5 displayed the same pathological phenotypes of_Adm–/–_ placentas — including (E) reduced fetal vessel branching in the labyrinth, as visualized by isolectin staining, (F) retention of SMCs around maternal SAs, and (G and H) significantly reduced number of DBA+ uNK cells — compared with_Calcrl+/+_ littermate placentas. *P < 0.001. n ≥ 6 placentas analyzed per genotype. Data are mean ± SEM. Original magnification, ×4 (A–C). Scale bars: 1 mm (D); 50 μM (E andG); 10 μM (F).
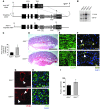
Figure 6. Genetic overexpression of fetal Adm reverses the placental preeclampsia phenotypes and drives uNK recruitment to the decidua.
(A) Targeting vector for generation of_Admhi/hi_ mice consisted of (a) a 6-kb genomic fragment of the Adm gene isolated from a 129S6/SvEv genomic phage library and containing all 4 exons and 5′UTR and 3′UTR of the Adm gene, (b) the bovine growth hormone polyA sequence (bGH 3′UTR), (c) 2 tandem copies of the 1.2-kb 5′ insulator sequence from chicken β-globin gene (2XIns), (d) 1.3 kb of pMC1 promoter–driven neomycin (Neo), (e) 80 bp of AU/U-rich element of the mouse c-fos gene (ARE), and (f) 2 loxP recombination sites. The latter 5 elements were cloned as a cassette, 23 bp downstream of the endogenous_Adm_ stop codon. (B) Southern blot analysis on genomic DNA confirmed correct targeting of the Admhi_allele. (C) Adm gene expression in placentas from_Adm+/+ and Admhi/hi_mice, analyzed by quantitative RT-PCR, showed a significant 3-fold increase in gene expression level. *P < 0.05. (D–G)Admhi/hi placentas (D) appeared histologically comparable to Adm+/+ placentas and showed (E) highly branched fetal labyrinth vessels, (F) appropriate SA remodeling, and (G) reendothelialization. (H and I) DBA staining revealed that_Admhi/hi placentas had a significant 30% increase in uNK cell numbers compared with _Adm+/+_littermate placentas. *P < 0.05. Data are mean ± SEM. Scale bars: 1 mm (D); 50 μM (E–H).
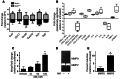
Figure 7. AM is a direct activator of uNK cells in vivo and in vitro.
(A) Using a mouse Chemokines and Receptors array platform (see Methods), the expression of 85 chemokines, cytokines, and related proteins was evaluated in placental RNA extracts from_Adm–/–_,Admhi/hi, and respective WT littermates.Admhi/hi placentas showed significant elevations in numerous cytokines and chemokines that were concomitantly downregulated in_Adm–/–_ placentas. *P < 0.05. (B) uNK cells isolated from E10.5–E12.5 WT placentas were cultured in vitro and treated with 10 nM AM. Screening for more than 25 chemokines and cytokines from media samples revealed significant 2- to 4-fold increases in secretion of numerous chemokines and cytokines and a prominent reduction in the secretion of CCL5, IL-16, and IL-23 in response to AM treatment. All changes were statistically significant compared with untreated control (P < 0.05). (C) Isolated WT uNK cells exhibited dose-dependent increases in _Mmp9_gene expression, which (D and E) correlated with increased MMP9 zymography activity. Mmp2 gene expression levels and zymography activity were not affected by AM treatment. *P< 0.05.
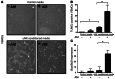
Figure 8. Conditioned media from AM-treated uNK cells causes VSMC apoptosis.
(A and B) Confluent monolayers of cultured mouse VSMCs were placed for 12 hours in control media or uNK-conditioned media treated or not with 10 nM AM peptide, then imaged (A) and stained with TUNEL (B). (C) After treatment with uNK-conditioned media, expression levels of the proapoptotic Bax and antiapoptotic_Bcl2_ genes were analyzed from VSMC RNA lysates by quantitative RT-PCR; data are expressed as Bax/Bcl2 ratio. *P < 0.05, 1-way ANOVA. Data are mean ± SEM. Original magnification, ×20 (A).
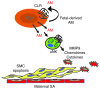
Figure 9. Model of fetal-derived AM action at the maternal-fetal interface.
AM and its G protein–coupled receptor, CLR, are highly expressed in parietal TGCs at the maternal-fetal interface at midgestation. The genetic dosage of fetal AM is directly proportional to the content of maternal uNK cells and their secreted chemokines, cytokines, and MMPs within the placenta. As a consequence, AM expression in fetal trophoblast cells is necessary for appropriate branching of the fetal vasculature and remodeling of maternal SAs. Overexpression of AM within the fetal compartment is sufficient to promote uNK recruitment and activation in the decidua, a process that likely contributes to AM-induced activation of MMP9 and maternal SA remodeling.
Similar articles
- Uterine natural killer cells as modulators of the maternal-fetal vasculature.
Matson BC, Caron KM. Matson BC, et al. Int J Dev Biol. 2014;58(2-4):199-204. doi: 10.1387/ijdb.140032kc. Int J Dev Biol. 2014. PMID: 25023686 Review. - An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array.
Yagel S, Cohen SM, Goldman-Wohl D. Yagel S, et al. Am J Obstet Gynecol. 2022 Feb;226(2S):S963-S972. doi: 10.1016/j.ajog.2020.10.023. Epub 2021 Mar 9. Am J Obstet Gynecol. 2022. PMID: 33712272 Review. - Uterine natural killer cells initiate spiral artery remodeling in human pregnancy.
Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, Baker PN, Robson SC, Bulmer JN. Robson A, et al. FASEB J. 2012 Dec;26(12):4876-85. doi: 10.1096/fj.12-210310. Epub 2012 Aug 23. FASEB J. 2012. PMID: 22919072 - Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta.
Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Adamson SL, et al. Dev Biol. 2002 Oct 15;250(2):358-73. doi: 10.1016/s0012-1606(02)90773-6. Dev Biol. 2002. PMID: 12376109 - Adrenomedullin and endocrine control of immune cells during pregnancy.
Matson BC, Caron KM. Matson BC, et al. Cell Mol Immunol. 2014 Sep;11(5):456-9. doi: 10.1038/cmi.2014.71. Epub 2014 Aug 18. Cell Mol Immunol. 2014. PMID: 25132453 Free PMC article. Review.
Cited by
- Adrenomedullin in lymphangiogenesis: from development to disease.
Klein KR, Caron KM. Klein KR, et al. Cell Mol Life Sci. 2015 Aug;72(16):3115-26. doi: 10.1007/s00018-015-1921-3. Epub 2015 May 8. Cell Mol Life Sci. 2015. PMID: 25953627 Free PMC article. Review. - Innate lymphoid cell sensing of tissue vitality.
Barrow AD, Colonna M. Barrow AD, et al. Curr Opin Immunol. 2019 Feb;56:82-93. doi: 10.1016/j.coi.2018.11.004. Epub 2018 Dec 5. Curr Opin Immunol. 2019. PMID: 30529190 Free PMC article. Review. - Uterine NK cells: active regulators at the maternal-fetal interface.
Moffett A, Colucci F. Moffett A, et al. J Clin Invest. 2014 May;124(5):1872-9. doi: 10.1172/JCI68107. Epub 2014 May 1. J Clin Invest. 2014. PMID: 24789879 Free PMC article. Review. - h_CALCRL_ mutation causes autosomal recessive nonimmune hydrops fetalis with lymphatic dysplasia.
Mackie DI, Al Mutairi F, Davis RB, Kechele DO, Nielsen NR, Snyder JC, Caron MG, Kliman HJ, Berg JS, Simms J, Poyner DR, Caron KM. Mackie DI, et al. J Exp Med. 2018 Sep 3;215(9):2339-2353. doi: 10.1084/jem.20180528. Epub 2018 Aug 16. J Exp Med. 2018. PMID: 30115739 Free PMC article. - Lymphatic Programing and Specialization in Hybrid Vessels.
Pawlak JB, Caron KM. Pawlak JB, et al. Front Physiol. 2020 Feb 20;11:114. doi: 10.3389/fphys.2020.00114. eCollection 2020. Front Physiol. 2020. PMID: 32153423 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD060860/HD/NICHD NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- R01 HD046970/HD/NICHD NIH HHS/United States
- HD46970/HD/NICHD NIH HHS/United States
- ARRA-HD060860-01S1/HD/NICHD NIH HHS/United States
- R01 HL109607/HL/NHLBI NIH HHS/United States
- R01 HL070953/HL/NHLBI NIH HHS/United States
- R01 HL091973/HL/NHLBI NIH HHS/United States
- HD060860/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases