Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH - PubMed (original) (raw)
Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH
Guo Zhang et al. Nature. 2013.
Abstract
Ageing is a result of gradual and overall functional deteriorations across the body; however, it is unknown whether an individual tissue primarily works to mediate the ageing progress and control lifespan. Here we show that the hypothalamus is important for the development of whole-body ageing in mice, and that the underlying basis involves hypothalamic immunity mediated by IκB kinase-β (IKK-β), nuclear factor κB (NF-κB) and related microglia-neuron immune crosstalk. Several interventional models were developed showing that ageing retardation and lifespan extension are achieved in mice by preventing ageing-related hypothalamic or brain IKK-β and NF-κB activation. Mechanistic studies further revealed that IKK-β and NF-κB inhibit gonadotropin-releasing hormone (GnRH) to mediate ageing-related hypothalamic GnRH decline, and GnRH treatment amends ageing-impaired neurogenesis and decelerates ageing. In conclusion, the hypothalamus has a programmatic role in ageing development via immune-neuroendocrine integration, and immune inhibition or GnRH restoration in the hypothalamus/brain represent two potential strategies for optimizing lifespan and combating ageing-related health problems.
Figures
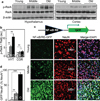
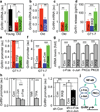
Figure 1. Aging-dependent hypothalamic NF-κB activation
57BL/6 mice (chow-fed males) were analyzed at young (3–4 months) age (Y), middle-old (11–13 months) age (M), and old (22–24 months) age (O). a&b. Hypothalami were analyzed via Western blots. b: Intensity of p-RelA normalized by RelA (au: arbitrary unit). c&d. Mice received MBH injections of lentiviral GFP controlled by NF-κB response element (NF-κB/RE), and following ~3-week recovery, brain sections were made to reveal GFP and NeuN stainining. DAPI staining shows entire cell populations. Bar = 25 µm. d: Percentages of cells co-expressing GFP and NeuN (GFP+NeuN+) among NeuN-expressing cells (NeuN+) in the MBH. **P < 0.01, ***P < 0.001; n = 6 (b) and 3 (d) per group. Error bars reflect mean ± SEM.
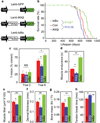
Figure 2. Aging manipulations by hypothalamic IKKβ/NF-κB
MBH-IKKβ, MBH-IκBα and MBH-Con mice were generated using ~18-month-old C57BL/6 mice (chow-fed males). a. Dual synapsin (Syn) promoter-directed lentiviral vectors. b. Lifespan of these mice (n = 23 – 31 mice per group). c&d. Mice at ~6 months post gene delivery were assessed for cognition via T-maze (c) and muscle endurance (d). e–h: Mice were scarified at 8~10 months post gene delivery for measuring muscle (quadriceps) fiber size (e), dermal thickness (f), bone mass (g), and tail tendon breaking time (h). *P < 0.05, **P < 0.01; MBH-Con: n = 23 (b), 9 (c), 6 (d), 3 (e&f), 4 (g), and 7 (h); MBH-IKKβ: n = 24 (b), 10 (c), 6 (d), 3 (e&f), 4 (g), and 5 (h); MBH-IκBα: n = 31 (b), 12 (c), 7 (d), 3 (e&f), 6 (g), and 8 (h). Error bars reflect mean ± SEM.
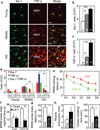
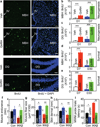
Figure 3. Role of hypothalamic microglia in aging
a–c Brain sections of young (Y), middle-old (M) and old (O) C57BL/6 mice were analyzed for hypothalamic microglia. a: Representative images of immunostaining. Bar = 25 µm. b&c: Numbers of cells expressing Iba-1 (Iba-1+) (b) or TNF-α (TNF-α+) (c) in the arcuate nucleus. d. Middle-old IKKβlox/lox mice received bilateral MBH injections of lentiviral CD11b promoter-driven Cre (CD11b) vs. control (Con). At 1 month vs. 8 months post injection, brain sections were made for Iba-1 and TNF-α staining (images in suppl. Fig. 4c). Mice generated at a young age provided normal references. Data show numbers of cells immunoreactive for Iba-1, TNF-α or both in the arcuate nucleus. e–i. Mice described in Fig. 3e were generated at a middle-old age and assessed at old ages for cognition (e–g), muscle endurance (h), and tail tendon breaking time (i). MWM data included time in target vs. a representative off-target (Off-T) quadrant in probe trials. *P < 0.05, **P < 0.01, ***P < 0.001; n = 4 (b&c) and 3 (d) per group; Con: n = 6 (e–g, i) and 9 (h); CD11b: n = 5 (e–g) and 6 (h&i). Error bars reflect mean ± SEM.
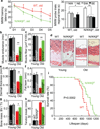
Figure 4. Genetic longevity by brain-specific IKKβ knockout
N/IKKβlox/lox mice (N/IKKβl/l) and littermate WT in males were maintained on a chow since weaning. a&b. Young (3 months) vs. old age (18–20 months) mice were tested for cognition (a) and muscle endurance (b). MWM data included time in target northwest (NW) vs. off-target northeast (NE), southwest (SW) and southeast (SE) quadrants in probe trials. c–h. Young (3–4 months) vs. old (20–24 months) mice were scarified for assessing muscle (quadriceps) fiber size (c), dermal thickness (d–f), bone mass (g), and tail tendon breaking time (h). i. Lifespan follow-up (n = 20 in WT and n = 25 in N/IKKβl/l). *P < 0.05, **P < 0.01; young WT: n = 10 (b), 3 (c&e), 5 (f), 6 (g) and 8 (h); young N/IKKβl/l: n = 14 (b), 3 (c,e,f), 6 (g) and 8 (h); old WT: n = 10 (a), 7 (b), 3 (c&e), 5 (f&g), and 6 (h); old N/IKKβl/l n = 10 (a), 7 (b), 3 (c,e,f), and 6 (g&h). Error bars reflect mean ± SEM.
Figure 5. Inhibition of GnRH by IKKβ/NF-κB
a–c Hypothalamic GnRH mRNA of indicated mice. d–g. GT1–7 cells were transfected with CAIKKβ, RelA or DNIκBα vs. control (Con) plasmid (d,e,g), co-transfected with _GnRH_-promoter luciferase plasmid (e&f), or together with RelA shRNA (sh-RelA) vs. control shRNA (sh-Con) plasmid (f), and were measure for GnRH release (d), GnRH promoter (e&f), and c-Fos, c-Jun, PKCα and PKCδ mRNA levels (g). h. GnRH promoter activities were measured for GT1–7 cells transfected with _GnRH_-promoter luciferase plasmid, co-transfected with c-Jun or c-Fos plasmid vs. control plasmid (Con), or treated with TPA vs. vehicle (Veh). i. GnRH promoter activities were measured for GT1–7 cells transfected with _GnRH_-promoter luciferase plasmid, co-transfected with CAIKKβ vs. control (Con) plasmid, and with c-Fos/c-Jun shRNA plasmids (sh-c-Fos/sh-c-Jun) vs. scramble shRNA control (sh-Con). j. Summarized schematic model. *P < 0.05, **P < 0.01, ***P < 0.001; n = 12 (a&e) and 3 (f–i) per group, and n = 6 (b), 8 (c) and 4 (d) in Con, n = 8 (b) and 6 (d) in IKKβ, and n = 8 (c) and 6 (d) in IκBα. Error bars reflect mean ± SEM.
Figure 6. Central and systemic actions of GnRH in counteracting aging
a–d C57BL/6 mice at an old age were subjected to neurogenesis (a&b) and survival (c&d) assays, as detailed in methods. a: BrdU staining of MBH and dentate gyrus (DG) in neurogenesis assay. Bar = 50 µm. b&c: BrdU-labeled cells (BrdU+) in the MBH (b) and DG (c) in neurogenesis assay. d&e: Survival of BrdU-labeled (BrdU+) cells in the MBH (d) and DG (e) in survival assay. f–i. MBH-IKKβ and MBH-Con mice at an old age were daily injected subcutaneously with GnRH vs. vehicle for 5 weeks, and analyzed for muscle endurance (f), skeletal muscle fibers**(g)**, skin (h), and cognition (i) (see suppl. Fig. 10 for additional data). *P < 0.05, **P < 0.01, ***P < 0.001; n = 4 (b–e), 7 (f), and 3 (g&h) per group, and n = 12 (Con, Veh), 7 (Con, GnRH), 7 (IKKβ, Veh), and 8 (IKKβ, GnRH) (i). Error bars reflect mean ± SEM.
Comment in
- Physiology: Inflammation links ageing to the brain.
Gabuzda D, Yankner BA. Gabuzda D, et al. Nature. 2013 May 9;497(7448):197-8. doi: 10.1038/nature12100. Epub 2013 May 1. Nature. 2013. PMID: 23636321 Free PMC article. No abstract available. - Hypothalamic inflammation and GnRH in aging development.
Tang Y, Cai D. Tang Y, et al. Cell Cycle. 2013 Sep 1;12(17):2711-2. doi: 10.4161/cc.26054. Epub 2013 Aug 19. Cell Cycle. 2013. PMID: 23966154 Free PMC article. No abstract available. - Can inflammation regulate systemic aging?
Patel P, Lockey RF, Kolliputi N. Patel P, et al. Exp Gerontol. 2015 Jul;67:1-2. doi: 10.1016/j.exger.2015.04.011. Epub 2015 Apr 23. Exp Gerontol. 2015. PMID: 25914111 No abstract available.
Similar articles
- Can inflammation regulate systemic aging?
Patel P, Lockey RF, Kolliputi N. Patel P, et al. Exp Gerontol. 2015 Jul;67:1-2. doi: 10.1016/j.exger.2015.04.011. Epub 2015 Apr 23. Exp Gerontol. 2015. PMID: 25914111 No abstract available. - β-amyloid-induced gonadotropin-releasing hormone decline involving Forkhead transcription factor FOXO3a and nuclear factor-κB.
Shi C, Shi R, Guo H, Shi Y, Liu X. Shi C, et al. Neuroreport. 2020 Aug 12;31(12):923-927. doi: 10.1097/WNR.0000000000001488. Neuroreport. 2020. PMID: 32658125 - Hypothalamic inflammation and GnRH in aging development.
Tang Y, Cai D. Tang Y, et al. Cell Cycle. 2013 Sep 1;12(17):2711-2. doi: 10.4161/cc.26054. Epub 2013 Aug 19. Cell Cycle. 2013. PMID: 23966154 Free PMC article. No abstract available. - Integrity of IKK/NF-κB Shields Thymic Stroma That Suppresses Susceptibility to Autoimmunity, Fungal Infection, and Carcinogenesis.
Zhu F, Hu Y. Zhu F, et al. Bioessays. 2018 Apr;40(4):e1700131. doi: 10.1002/bies.201700131. Epub 2018 Mar 9. Bioessays. 2018. PMID: 29522649 Review. - Proteins that bind to IKKgamma (NEMO) and down-regulate the activation of NF-kappaB.
Shifera AS. Shifera AS. Biochem Biophys Res Commun. 2010 Jun 4;396(3):585-9. doi: 10.1016/j.bbrc.2010.05.012. Epub 2010 May 10. Biochem Biophys Res Commun. 2010. PMID: 20457134 Review.
Cited by
- The impact of inactivation of the GH/IGF axis during aging on healthspan.
Poudel SB, Ruff RR, He Z, Dixit M, Yildirim G, Jayarathne H, Manchanayake DH, Basta-Pljakic J, Duran-Ortiz S, Schaffler MB, Kopchick JJ, Sadagurski M, Yakar S. Poudel SB, et al. Geroscience. 2024 Nov 13. doi: 10.1007/s11357-024-01426-3. Online ahead of print. Geroscience. 2024. PMID: 39535693 - Targeting the hypothalamus for modeling age-related DNA methylation and developing OXT-GnRH combinational therapy against Alzheimer's disease-like pathologies in male mouse model.
Usmani SS, Jung HG, Zhang Q, Kim MW, Choi Y, Caglayan AB, Cai D. Usmani SS, et al. Nat Commun. 2024 Oct 31;15(1):9419. doi: 10.1038/s41467-024-53507-8. Nat Commun. 2024. PMID: 39482312 Free PMC article. - Targeting immunosenescence for improved tumor immunotherapy.
Liu Z, Zuo L, Zhou Z, Liu S, Ba Y, Zuo A, Ren Y, Zhang C, Chen Y, Ma H, Xu Y, Luo P, Cheng Q, Xu H, Zhang Y, Weng S, Han X. Liu Z, et al. MedComm (2020). 2024 Oct 28;5(11):e777. doi: 10.1002/mco2.777. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39473905 Free PMC article. Review. - Hypothalamic SLC7A14 accounts for aging-reduced lipolysis in white adipose tissue of male mice.
Jiang X, Liu K, Luo P, Li Z, Xiao F, Jiang H, Wu S, Tang M, Yuan F, Li X, Shu Y, Peng B, Chen S, Ni S, Guo F. Jiang X, et al. Nat Commun. 2024 Sep 11;15(1):7948. doi: 10.1038/s41467-024-52059-1. Nat Commun. 2024. PMID: 39261456 Free PMC article. - Role of the central nervous system in cell non-autonomous signaling mechanisms of aging and longevity in mammals.
Urushihata T, Satoh A. Urushihata T, et al. J Physiol Sci. 2024 Aug 31;74(1):40. doi: 10.1186/s12576-024-00934-3. J Physiol Sci. 2024. PMID: 39217308 Free PMC article. Review.
References
Reference List
- Masoro EJ. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005;126:913–922. - PubMed
- Finch CE. Neurons, glia, and plasticity in normal brain aging. Adv. Gerontol. 2002;10:35–39. - PubMed
- Zitnik G, Martin GM. Age-related decline in neurogenesis: old cells or old environment? J. Neurosci. Res. 2002;70:258–263. - PubMed
Supplementary Reference List
- Tillerson JL, Miller GW. Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of parkinsonism. J. Neurosci. Methods. 2003;123:189–200. - PubMed
- Mueller JM, Pahl HL. Assaying NF-kappa B and AP-1 DNA-binding and transcriptional activity. Methods Mol. Biol. 2000;99:205–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials