LKB1 represses focal adhesion kinase (FAK) signaling via a FAK-LKB1 complex to regulate FAK site maturation and directional persistence - PubMed (original) (raw)
LKB1 represses focal adhesion kinase (FAK) signaling via a FAK-LKB1 complex to regulate FAK site maturation and directional persistence
Erik R Kline et al. J Biol Chem. 2013.
Abstract
Liver kinase β1 (LKB1, also known as STK11) is a serine/threonine kinase that has multiple cellular functions including the regulation of cell polarity and motility. Murine proteomic studies show that LKB1 loss causes aberrant adhesion signaling; however, the mechanistic underpinnings of this relationship are unknown. We show that cells stably depleted of LKB1 or its co-activator STRADα have increased phosphorylation of focal adhesion kinase (FAK) at Tyr(397)/Tyr(861) and enhanced adhesion to fibronectin. LKB1 associates in a complex with FAK and LKB1 accumulation at the cellular leading edge is mutually excluded from regions of activated Tyr(397)-FAK. LKB1-compromised cells lack directional persistence compared with wild-type cells, but this is restored through subsequent pharmacological FAK inhibition or depletion, showing that cell directionality is mediated through LKB1-FAK signaling. Live cell confocal imaging reveals that LKB1-compromised cells lack normal FAK site maturation and turnover, suggesting that defects in adhesion and directional persistence are caused by aberrant adhesion dynamics. Furthermore, re-expression of full-length wild-type or the LKB1 N-terminal domain repressed FAK activity, whereas the kinase domain or C-terminal domain alone did not, indicating that FAK suppression is potentially regulated through the LKB1 N-terminal domain. Based upon these results, we conclude that LKB1 serves as a FAK repressor to stabilize focal adhesion sites, and when LKB1 function is compromised, aberrant FAK signaling ensues, resulting in rapid FAK site maturation and poor directional persistence.
Keywords: Adhesion; Cell Migration; Cell Motility; Epithelial Mesenchymal Transition; Focal Adhesion Kinase; Imaging; LKB1.
Figures
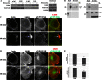
FIGURE 1.
Compromised LKB1 function increases FAK phosphorylation and enhances cell adhesion. A, knockdown of either LKB1 or STRAD results in elevated levels of both Tyr(P)861- and Tyr(P)397-FAK by Western blot, indicating enhanced FAK activity in these cells when on fibronectin. B, representative images of the leading edge of motile pLKO.1 and LKB1 shRNA cells demonstrate that fluorescence intensities of both Tyr(P)861- and Tyr(P)397-FAK are elevated when cells are stably depleted of LKB1. C, bar graphs quantitating mean Tyr(P)397 FAK site width and the peak relative fluorescence intensity of Tyr(P)397 FAK sites in pLKO.1 control and LKB1 shRNA cells (***, p < 0.001). D, a cell adhesion assay shows that by 20 min post-seeding, there are significantly more H1299 cells attached to the fibronectin-coated tissue culture plate when cells are stably depleted of either LKB1 or the LKB1 co-activating binding partner STRADα compared with wild-type LKB1-expressing pLKO.1 cells. *, p < 0.05 versus pLKO.1. Data are shown as mean ± S.E.
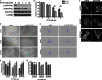
FIGURE 2.
LKB1 and FAK associate in a complex that is dependent upon the LKB1 N-terminal domain. A, co-immunoprecipitation experiments show that wild-type LKB1 from control cells pulls down total FAK protein, and that total FAK pull-down conversely co-immunoprecipitates LKB1. Species- and isotype-specific IgG antibodies were used as negative controls. B, in vitro binding assay using purified FAK and purified LKB1-STRAD-MO25 complex. Either purified LKB1 or FAK were immunoprecipitated using the respective antibody and then probed by Western blot for LKB1 or FAK. Left blots show IP:LKB1 and right blots IP:FAK. In both cases the binding partner was not present by Western blot above background levels. Note that in the IP:FAK experiment (rightmost blot) the LKB1 signal (3rd lane) is not greater than background (2nd lane). C and D, confocal immunofluorescence images of Tyr(P)397-FAK and Tyr(P)861-FAK at 20 and 60 min post wounding. Light blue arrows point to regions of LKB1 accumulation and white arrowheads indicate regions of either Tyr(P)397-FAK (C) or Tyr(P)861-FAK (D) accumulation. Scale bar = 10 μm. E, bar graphs showing Tyr(P)397-FAK (top) and Tyr(P)861-FAK (bottom) intensity at leading edge regions containing LKB1 (+LKB1) compared with regions where LKB1 is absent (−LKB1; ***, p < 0.005).
FIGURE 3.
Pharmacologically targeting FAK with PF-573228 restores directional persistence in LKB1-compromised cells. A, Western blot of Tyr(P)397-FAK and Tyr(P)861-FAK after increasing concentrations of PF-573228. DMSO was used as a control. B, bar graph showing that the FAK inhibitor dose-dependently decreases cell adhesion in control pLKO.1 cells and more potently inhibits adhesion in LKB1-depleted cells at 1 and 5 μ
m
. **, p < 0.01. DMSO was used as a control. Data are shown as mean ± S.E. Data were normalized separately for each experimental group to 100% in untreated cells, to adequately compare pLKO.1 versus shLKB1. C, confocal images of immunofluorescence staining of Tyr(P)397-FAK sites in control, shLKB1, and shSTRAD cells after PF-573228 treatment. Inset shows a magnified region of cellular leading edge. Scale bar = 5 μm. D, representative traces and E, migratory plot diagrams show that shLKB1 and shSTRAD H1299 cells have poor directionality and circuitous migration paths compared with pLKO.1 cells, which is reversed upon treatment with the small molecular FAK inhibitor. PF-573228 dose-dependently improves directionality. F, bar graph of the meandering indices in pLKO.1 control, shLKB1, and shSTRAD cells with and without PF-573228 treatment. G, bar graph of cellular velocities in pLKO.1 control, shLKB1, and shSTRAD cells with and without PF-573228 treatment. In F and G, *, p < 0.05; **, p < 0.01; NS, not significant versus 0 μ
m
PF-573228 group.
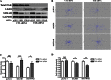
FIGURE 4.
FAK knockdown restores directional persistence in LKB1-compromised cells. A, FAK-specific siRNA was used to successfully knockdown total FAK protein levels by Western blot. B, migratory plot diagrams from live-cell confocal microscopy experiments demonstrate that FAK siRNA transfection increases the directionality and migration track lengths in shLKB1 and shSTRAD H1299 cells compared with control siRNA-treated cells. C, meandering index analysis quantitatively shows that transient FAK knockdown restores directionality in LKB1-compromised cells and D, concomitantly decreases cell velocity. *, p < 0.05; ***, p < 0001; NS, not significant, n = 40+.
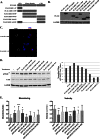
FIGURE 5.
LKB1 re-expression suppresses FAK likely through the LKB1 N-terminal domain. A, diagram of FLAG-tagged LKB1 truncates illustrates the amino acids and domains present in each construct. CTD, carboxyl-terminal domain. B, FLAG-tagged LKB1 truncates were transfected into LKB1-null H157 NSCLC cells, and expression was verified by Western blotting. C, single confocal section of immunofluorescence for FLAG in cells transfected with WT-LKB1 or FLAG-LKB1-(1–47). D, Western blot of Tyr(P)397-FAK after transfection of LKB1 truncates into H157 cells and densitometry on left. Bars represent mean Tyr(P)397-FAK gray levels after normalization to total FAK levels. E, bar graph showing the mean meandering indices of cells that have the GFP:LKB1 truncates or GFP only control (* = p < 0.05; **, p < 0.005; ***, p < 0.0005 compared with GFP only cells). Error bars show ±S.D. E, bar graph showing the mean velocity of cells that have the GFP:LKB1 truncates or GFP only control (*, p < 0.05; **, p < 0.005; ***, p < 0.0005 compared with GFP only cells). Error bars show ±S.D.
FIGURE 6.
LKB1 loss causes rapid focal adhesion site turnover. GFP-FAK was overexpressed in either pLKO.1 or shLKB1 H1299 cells to image focal adhesion sites by live-cell confocal microscopy. A, single frames from a live cell confocal time lapse of GFP:FAK in pLKO.1 control and shLKB1 cells. B, individual focal adhesions are significantly more transient in cells depleted of LKB1 compared with control cells. ***, p < 0.0001. Scale bars = 4 μm in A and B. C, representative focal adhesion life plots show that GFP-FAK sites are shorter-lived when LKB1 is depleted, and that the (D) initial FAK site growth is less when LKB1 is depleted. E, bar graphs showing that the mean size of individual GFP-FAK sites is ∼1.5-fold smaller in LKB1-depleted cells compared with control pLKO.1 cells. ***, p < 0.0001, n = 25. Error bars show ±S.D.
Similar articles
- Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility.
Wang W, Liu Y, Liao K. Wang W, et al. BMC Cell Biol. 2011 Nov 13;12:49. doi: 10.1186/1471-2121-12-49. BMC Cell Biol. 2011. PMID: 22078467 Free PMC article. - Coordinated cell motility is regulated by a combination of LKB1 farnesylation and kinase activity.
Wilkinson S, Hou Y, Zoine JT, Saltz J, Zhang C, Chen Z, Cooper LA, Marcus AI. Wilkinson S, et al. Sci Rep. 2017 Jan 19;7:40929. doi: 10.1038/srep40929. Sci Rep. 2017. PMID: 28102310 Free PMC article. - Therapeutic Potential of Focal Adhesion Kinase Inhibition in Small Cell Lung Cancer.
Aboubakar Nana F, Lecocq M, Ladjemi MZ, Detry B, Dupasquier S, Feron O, Massion PP, Sibille Y, Pilette C, Ocak S. Aboubakar Nana F, et al. Mol Cancer Ther. 2019 Jan;18(1):17-27. doi: 10.1158/1535-7163.MCT-18-0328. Epub 2018 Oct 23. Mol Cancer Ther. 2019. PMID: 30352800 Free PMC article. - Control of motile and invasive cell phenotypes by focal adhesion kinase.
Schlaepfer DD, Mitra SK, Ilic D. Schlaepfer DD, et al. Biochim Biophys Acta. 2004 Jul 5;1692(2-3):77-102. doi: 10.1016/j.bbamcr.2004.04.008. Biochim Biophys Acta. 2004. PMID: 15246681 Review. - New insights into FAK structure and function in focal adhesions.
Le Coq J, Acebrón I, Rodrigo Martin B, López Navajas P, Lietha D. Le Coq J, et al. J Cell Sci. 2022 Oct 15;135(20):jcs259089. doi: 10.1242/jcs.259089. Epub 2022 Oct 14. J Cell Sci. 2022. PMID: 36239192 Review.
Cited by
- Ezrin regulates focal adhesion and invadopodia dynamics by altering calpain activity to promote breast cancer cell invasion.
Hoskin V, Szeto A, Ghaffari A, Greer PA, Côté GP, Elliott BE. Hoskin V, et al. Mol Biol Cell. 2015 Oct 1;26(19):3464-79. doi: 10.1091/mbc.E14-12-1584. Epub 2015 Aug 5. Mol Biol Cell. 2015. PMID: 26246600 Free PMC article. - LKB1 deficiency promotes proliferation and invasion of glioblastoma through activation of mTOR and focal adhesion kinase signaling pathways.
Zhang K, Wang J, Wang J, Luh F, Liu X, Yang L, Liu YR, Su L, Yang YS, Chu P, Yen Y. Zhang K, et al. Am J Cancer Res. 2019 Aug 1;9(8):1650-1663. eCollection 2019. Am J Cancer Res. 2019. PMID: 31497348 Free PMC article. - Mechanisms of environmental chemicals that enable the cancer hallmark of evasion of growth suppression.
Nahta R, Al-Mulla F, Al-Temaimi R, Amedei A, Andrade-Vieira R, Bay SN, Brown DG, Calaf GM, Castellino RC, Cohen-Solal KA, Colacci A, Cruickshanks N, Dent P, Di Fiore R, Forte S, Goldberg GS, Hamid RA, Krishnan H, Laird DW, Lasfar A, Marignani PA, Memeo L, Mondello C, Naus CC, Ponce-Cusi R, Raju J, Roy D, Roy R, Ryan EP, Salem HK, Scovassi AI, Singh N, Vaccari M, Vento R, Vondráček J, Wade M, Woodrick J, Bisson WH. Nahta R, et al. Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S2-18. doi: 10.1093/carcin/bgv028. Carcinogenesis. 2015. PMID: 26106139 Free PMC article. Review. - Live-Cell Invasive Phenotyping Uncovers ALK2 as a Therapeutic Target in LKB1-Mutant Lung Cancer.
Koo J, Seong CS, Parker RE, Herrera A, Dwivedi B, Arthur RA, Dinasarapu AR, Johnston HR, Claussen H, Tucker-Burden C, Ramalingam SS, Fu H, Zhou W, Marcus AI, Gilbert-Ross M. Koo J, et al. Cancer Res. 2024 Nov 15;84(22):3761-3771. doi: 10.1158/0008-5472.CAN-23-2631. Cancer Res. 2024. PMID: 39207369 Free PMC article. - Targeting focal adhesion kinase boosts immune response in KRAS/LKB1 co-mutated lung adenocarcinoma via remodeling the tumor microenvironment.
Qiao M, Zhou F, Liu X, Jiang T, Wang H, Li X, Zhao C, Cheng L, Chen X, Ren S, Wang Z, Zhou C. Qiao M, et al. Exp Hematol Oncol. 2024 Jan 30;13(1):11. doi: 10.1186/s40164-023-00471-6. Exp Hematol Oncol. 2024. PMID: 38291516 Free PMC article.
References
- Bai X. M., Zhang W., Liu N. B., Jiang H., Lou K. X., Peng T., Ma J., Zhang L., Zhang H., Leng J. (2009) Focal adhesion kinase. Important to prostaglandin E2-mediated adhesion, migration and invasion in hepatocellular carcinoma cells. Oncol. Rep. 21, 129–136 - PubMed
- Fiorilli P., Partridge D., Staniszewska I., Wang J. Y., Grabacka M., So K., Marcinkiewicz C., Reiss K., Khalili K., Croul S. E. (2008) Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Lab. Invest. 88, 1143–1156 - PMC - PubMed
- Raveh S., Gavert N., Ben-Ze'ev A. (2009) L1 cell adhesion molecule (L1CAM) in invasive tumors. Cancer Lett. 282, 137–145 - PubMed
- Slack-Davis J. K., Atkins K. A., Harrer C., Hershey E. D., Conaway M. (2009) Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 69, 1469–1476 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA116676/CA/NCI NIH HHS/United States
- R01 CA142858/CA/NCI NIH HHS/United States
- 1R01CA142858/CA/NCI NIH HHS/United States
- 3P01CA116676-05S2/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous