The susceptibility of Pseudomonas aeruginosa strains from cystic fibrosis patients to bacteriophages - PubMed (original) (raw)
The susceptibility of Pseudomonas aeruginosa strains from cystic fibrosis patients to bacteriophages
Christiane Essoh et al. PLoS One. 2013.
Abstract
Phage therapy may become a complement to antibiotics in the treatment of chronic Pseudomonas aeruginosa infection. To design efficient therapeutic cocktails, the genetic diversity of the species and the spectrum of susceptibility to bacteriophages must be investigated. Bacterial strains showing high levels of phage resistance need to be identified in order to decipher the underlying mechanisms. Here we have selected genetically diverse P. aeruginosa strains from cystic fibrosis patients and tested their susceptibility to a large collection of phages. Based on plaque morphology and restriction profiles, six different phages were purified from "pyophage", a commercial cocktail directed against five different bacterial species, including P. aeruginosa. Characterization of these phages by electron microscopy and sequencing of genome fragments showed that they belong to 4 different genera. Among 47 P. aeruginosa strains, 13 were not lysed by any of the isolated phages individually or by pyophage. We isolated two new phages that could lyse some of these strains, and their genomes were sequenced. The presence/absence of a CRISPR-Cas system (Clustered Regularly Interspaced Short Palindromic Repeats and Crisper associated genes) was investigated to evaluate the role of the system in phage resistance. Altogether, the results show that some P. aeruginosa strains cannot support the growth of any of the tested phages belonging to 5 different genera, and suggest that the CRISPR-Cas system is not a major defence mechanism against these lytic phages.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Morphology of plaques produced by three different phages of the pyophage A) P1-15pyo on PAO1, B) P1-14pyo on C1-14, C) PTr60pyo on Tr60.
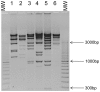
Figure 2. Restriction analysis of phage DNA.
The DNA of each of six phages from pyophage was digested by _Hind_III and electrophorezed on a 0.8% agarose gel. From 1 to 6: P1-15pyo, P8-13pyo, P2-10pyo, P3-20pyo, PTr60pyo, P1-14pyo. MW is a size marker. On the side are indicated the sizes of three DNA fragments. The DNA of P1-14pyo is not totally digested.
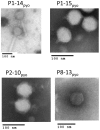
Figure 3. Electron microscopy observation of four phages from pyophage: P8-13pyo, P1-14pyo, P1-15pyo, P2-10pyo.
Figure 4. Electron microscopy observation of the new phages P1-14Or01 and P2-10Ab01.
Figure 5. Alignment of P1-14Or01, MR199-2 and LUZ24 genomes.
Putative open reading frames (ORF) are shown with arrows. In red are shown the tentative cluster of genes encoding structural proteins and in yellow the genes involved in DNA replication. tRNA genes are shown with triangles. Boxes with different shades of grey represent degrees of similarities between genomes.
Figure 6. Alignment of P2-10Ab01, JG004 and PAKP1 genomes.
The genomes are separated into two sections (A and B) of approximately 46 kb. The legend is that of Figure 4.
Similar articles
- Design of a Broad-Range Bacteriophage Cocktail That Reduces Pseudomonas aeruginosa Biofilms and Treats Acute Infections in Two Animal Models.
Forti F, Roach DR, Cafora M, Pasini ME, Horner DS, Fiscarelli EV, Rossitto M, Cariani L, Briani F, Debarbieux L, Ghisotti D. Forti F, et al. Antimicrob Agents Chemother. 2018 May 25;62(6):e02573-17. doi: 10.1128/AAC.02573-17. Print 2018 Jun. Antimicrob Agents Chemother. 2018. PMID: 29555626 Free PMC article. - In vitro and in vivo antibacterial activity of environmental bacteriophages against Pseudomonas aeruginosa strains from cystic fibrosis patients.
Olszak T, Zarnowiec P, Kaca W, Danis-Wlodarczyk K, Augustyniak D, Drevinek P, de Soyza A, McClean S, Drulis-Kawa Z. Olszak T, et al. Appl Microbiol Biotechnol. 2015 Jul;99(14):6021-33. doi: 10.1007/s00253-015-6492-6. Epub 2015 Mar 12. Appl Microbiol Biotechnol. 2015. PMID: 25758956 Free PMC article. - Pseudomonas aeruginosa Bacteriophages and Their Clinical Applications.
Alipour-Khezri E, Skurnik M, Zarrini G. Alipour-Khezri E, et al. Viruses. 2024 Jun 29;16(7):1051. doi: 10.3390/v16071051. Viruses. 2024. PMID: 39066214 Free PMC article. Review. - Current knowledge in the use of bacteriophages to combat infections caused by Pseudomonas aeruginosa in cystic fibrosis.
Martínez-Gallardo MJ, Villicaña C, Yocupicio-Monroy M, Alcaraz-Estrada SL, León-Félix J. Martínez-Gallardo MJ, et al. Folia Microbiol (Praha). 2023 Feb;68(1):1-16. doi: 10.1007/s12223-022-00990-5. Epub 2022 Aug 5. Folia Microbiol (Praha). 2023. PMID: 35931928 Review.
Cited by
- Identification and characterization of phage protein and its activity against two strains of multidrug-resistant Pseudomonas aeruginosa.
Al-Wrafy F, Brzozowska E, Górska S, Drab M, Strus M, Gamian A. Al-Wrafy F, et al. Sci Rep. 2019 Sep 17;9(1):13487. doi: 10.1038/s41598-019-50030-5. Sci Rep. 2019. PMID: 31530875 Free PMC article. - Identification of bacteriophages for biocontrol of the kiwifruit canker phytopathogen Pseudomonas syringae pv. actinidiae.
Frampton RA, Taylor C, Holguín Moreno AV, Visnovsky SB, Petty NK, Pitman AR, Fineran PC. Frampton RA, et al. Appl Environ Microbiol. 2014 Apr;80(7):2216-28. doi: 10.1128/AEM.00062-14. Epub 2014 Jan 31. Appl Environ Microbiol. 2014. PMID: 24487530 Free PMC article. - Characterization of the Newly Isolated Lytic Bacteriophages KTN6 and KT28 and Their Efficacy against Pseudomonas aeruginosa Biofilm.
Danis-Wlodarczyk K, Olszak T, Arabski M, Wasik S, Majkowska-Skrobek G, Augustyniak D, Gula G, Briers Y, Jang HB, Vandenheuvel D, Duda KA, Lavigne R, Drulis-Kawa Z. Danis-Wlodarczyk K, et al. PLoS One. 2015 May 21;10(5):e0127603. doi: 10.1371/journal.pone.0127603. eCollection 2015. PLoS One. 2015. PMID: 25996839 Free PMC article. - A novel lytic phage exhibiting a remarkable in vivo therapeutic potential and higher antibiofilm activity against Pseudomonas aeruginosa.
Abdelghafar A, El-Ganiny A, Shaker G, Askoura M. Abdelghafar A, et al. Eur J Clin Microbiol Infect Dis. 2023 Oct;42(10):1207-1234. doi: 10.1007/s10096-023-04649-y. Epub 2023 Aug 23. Eur J Clin Microbiol Infect Dis. 2023. PMID: 37608144 Free PMC article. - Production of Inhalation Phage Powders Using Spray Freeze Drying and Spray Drying Techniques for Treatment of Respiratory Infections.
Leung SS, Parumasivam T, Gao FG, Carrigy NB, Vehring R, Finlay WH, Morales S, Britton WJ, Kutter E, Chan HK. Leung SS, et al. Pharm Res. 2016 Jun;33(6):1486-96. doi: 10.1007/s11095-016-1892-6. Epub 2016 Feb 29. Pharm Res. 2016. PMID: 26928668 Free PMC article.
References
- Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, et al. (1995) Common virulence factors for bacterial pathogenicity in plants and animals. Science 268: 1899–1902. - PubMed
- Davies JC, Bilton D (2009) Bugs, biofilms, and resistance in cystic fibrosis. Respir Care 54: 628–640. - PubMed
- Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, et al. (2010) Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 11: 69–86. - PubMed
Publication types
MeSH terms
Grants and funding
This study was performed with the financial support of the association Vaincre La Mucoviscidose (Grant N° 2010/IC1020). The development of tools for the surveillance of bacterial pathogens is supported by the French Direction Générale de l'Armement (DGA). CE holds a fellowship of Agence Universitaire de la Francophonie. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical