Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity - PubMed (original) (raw)
Comparative Study
Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity
Cristina d'Abramo et al. PLoS One. 2013.
Abstract
The use of antibodies to treat neurodegenerative diseases has undergone rapid development in the past decade. To date, immunotherapeutic approaches to Alzheimer's disease have mostly targeted amyloid beta as it is a secreted protein that can be found in plasma and CSF and is consequently accessible to circulating antibodies. Few recent publications have suggested the utility of treatment of tau pathology with monoclonal antibodies to tau. Our laboratory has begun a systematic study of different classes of tau monoclonal antibodies using mutant P301L mice. Three or seven months old mutant tau mice were inoculated weekly with tau monoclonal antibodies at a dose of 10 mg/Kg, until seven or ten months of age were reached respectively. Our data strongly support the notion that in P301L animals treated with MC1, a conformational monoclonal antibody specific for PHF-tau, the rate of development of tau pathology is effectively reduced, while injecting DA31, a high affinity tau sequence antibody, does not exert such benefit. MC1 appears superior to DA31 in overall effects, suggesting that specificity is more important than affinity in therapeutic applications. Unfortunately the survival rate of the P301L treated mice was not improved when immunizing either with MC1 or PHF1, a high affinity phospho-tau antibody previously reported to be efficacious in reducing pathological tau. These data demonstrate that passive immunotherapy in mutant tau models may be efficacious in reducing the development of tau pathology, but a great deal of work remains to be done to carefully select the tau epitopes to target.
Conflict of interest statement
Competing Interests: Supported by a grant from Eli Lilly and Co. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures
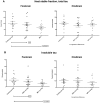
Figure 1. Relative affinities of tau monoclonal antibodies.
PHF-tau (0.5 µg/ml) purified from Alzheimer brain was used in ELISA in order to determine the relative binding abilities of a battery of tau monoclonal antibodies. In this assay, DA31 and PHF1 have much higher relative affinities for PHF-tau than does MC1.
Figure 2. P301L mice (n = 13–15 per group) immunized with MC1 or DA31, 3 to 7 months of age.
a) MC1-treated mice display a significant reductions (p = 0.023) in forebrain total tau, while DA31-treated mice do not differ from controls. b) When injecting MC1 the mice exhibit a significant decrease (p = 0.022) in forebrain insoluble pathological tau, and again DA31 fails in exerting any beneficial effect. No significant differences were detected when analyzing the hindbrains of treated versus aged-matched controls (a–b).
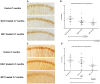
Figure 3. Representative CP13 and RZ3 immunohistochemistry of immunized P301L mice, 3 to 7 months of age.
The CA1 hippocampal region of either MC1 or DA31 treated mice was stained with two different phospho-tau antibodies. A–b) CP13 (pSer202) staining is significantly reduced (p = 0.0018) after injecting MC1 or DA31 (p = 0.046). c-d) RZ3 (pThr231) immunoreactivity displays a significant decrease (p = 0.002) in mice treated with MC1, while DA31 does not show any obvious changes. Results are expressed as percent of area stained.
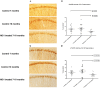
Figure 4. Representative CP13 and RZ3 immunohistochemistry of immunized P301L mice, 7 to 10 months of age.
The CA1 hippocampal region of the MC1 treated mice was stained with two different phospho-tau antibodies. a–b) CP13 (pSer202) staining is significantly reduced after injecting MC1, both when comparing 7 and 10 months old controls mice with MC1 treated animals from 7 to 10 months of age (p = 0.016 and p = 0.036 respectively). c–d) RZ3 (pThr231) immunoreactivity displays a significant decrease in mice treated with MC1 from 7 to 10 months of age (p = 0.013). Results are expressed as percent of area stained.
Figure 5. P301L mice (n = 13–15 per group) immunized with MC1, 7 to 10 months of age.
a) Insoluble tau analysis, using the DA31-DA9hrp ELISA, does not show any obvious change both in forebrain and hindbrain of treated mice. b) In forebrain, the ratios of insoluble pS202 and pThr231 over total tau are significantly reduced (p = 0.0145, p = 0.0196). The hindbrain analysis does not show any effect.
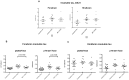
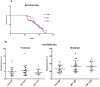
Figure 6. Survival experiment.
a) P301L mice were treated with MC1, PHF1 or saline from 7 months of age (200 days): no difference in the survival rate is observed between the animal cohorts. b) The analysis of forebrain and hindbrain insoluble tau does not show any difference between groups, correlating well with the survival rate data.
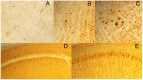
Figure 7. IgG do not enter into neuronal cell bodies.
A) Goat anti-mouse IgG1 followed by Streptavidin-HRP staining: some immunoreactivity from the blood vessels, but no detectable staining of neurons. B–C) PG5 (pSer409) staining of brain stem of P301L mice: neuronal staining is readily detectable when present. D) Biotinylated-secondary antibody followed by Streptavidin-HRP staining: no neuronal reactivity is seen. Blood vessels staining is detectable in the hippocampus of the mice. E) The same mouse does show any tau pathology (CP13) in hippocampal pyramidal cells.
Similar articles
- Tau-targeting passive immunization modulates aspects of pathology in tau transgenic mice.
Ittner A, Bertz J, Suh LS, Stevens CH, Götz J, Ittner LM. Ittner A, et al. J Neurochem. 2015 Jan;132(1):135-45. doi: 10.1111/jnc.12821. Epub 2014 Aug 1. J Neurochem. 2015. PMID: 25041093 - Vectored Intracerebral Immunization with the Anti-Tau Monoclonal Antibody PHF1 Markedly Reduces Tau Pathology in Mutant Tau Transgenic Mice.
Liu W, Zhao L, Blackman B, Parmar M, Wong MY, Woo T, Yu F, Chiuchiolo MJ, Sondhi D, Kaminsky SM, Crystal RG, Paul SM. Liu W, et al. J Neurosci. 2016 Dec 7;36(49):12425-12435. doi: 10.1523/JNEUROSCI.2016-16.2016. J Neurosci. 2016. PMID: 27927959 Free PMC article. - Passive Immunization in JNPL3 Transgenic Mice Using an Array of Phospho-Tau Specific Antibodies.
d'Abramo C, Acker CM, Jimenez H, Davies P. d'Abramo C, et al. PLoS One. 2015 Aug 13;10(8):e0135774. doi: 10.1371/journal.pone.0135774. eCollection 2015. PLoS One. 2015. PMID: 26270821 Free PMC article. - Tau Immunotherapy.
Sigurdsson EM. Sigurdsson EM. Neurodegener Dis. 2016;16(1-2):34-8. doi: 10.1159/000440842. Epub 2015 Nov 10. Neurodegener Dis. 2016. PMID: 26551002 Free PMC article. Review. - Passive Immunotherapies Targeting Amyloid Beta and Tau Oligomers in Alzheimer's Disease.
Vander Zanden CM, Chi EY. Vander Zanden CM, et al. J Pharm Sci. 2020 Jan;109(1):68-73. doi: 10.1016/j.xphs.2019.10.024. Epub 2019 Oct 21. J Pharm Sci. 2020. PMID: 31647950 Free PMC article. Review.
Cited by
- Targeting Prion-like Cis Phosphorylated Tau Pathology in Neurodegenerative Diseases.
Albayram O, Angeli P, Bernstein E, Baxley S, Gao Z, Lu KP, Zhou XZ. Albayram O, et al. J Alzheimers Dis Parkinsonism. 2018;8(3):443. doi: 10.4172/2161-0460.1000443. Epub 2018 Jun 29. J Alzheimers Dis Parkinsonism. 2018. PMID: 30197831 Free PMC article. - Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody.
Albert M, Mairet-Coello G, Danis C, Lieger S, Caillierez R, Carrier S, Skrobala E, Landrieu I, Michel A, Schmitt M, Citron M, Downey P, Courade JP, Buée L, Colin M. Albert M, et al. Brain. 2019 Jun 1;142(6):1736-1750. doi: 10.1093/brain/awz100. Brain. 2019. PMID: 31038156 Free PMC article. - Active immunization with tau epitope in a mouse model of tauopathy induced strong antibody response together with improvement in short memory and pSer396-tau pathology.
Joly-Amado A, Davtyan H, Serraneau K, Jules P, Zitnyar A, Pressman E, Zagorski K, Antonyan T, Hovakimyan A, Paek HJ, Gordon MN, Cribbs DH, Petrovsky N, Agadjanyan MG, Ghochikyan A, Morgan D. Joly-Amado A, et al. Neurobiol Dis. 2020 Feb;134:104636. doi: 10.1016/j.nbd.2019.104636. Epub 2019 Oct 17. Neurobiol Dis. 2020. PMID: 31629891 Free PMC article. - Abolishing Tau cleavage by caspases at Aspartate421 causes memory/synaptic plasticity deficits and pre-pathological Tau alterations.
Biundo F, d'Abramo C, Tambini MD, Zhang H, Del Prete D, Vitale F, Giliberto L, Arancio O, D'Adamio L. Biundo F, et al. Transl Psychiatry. 2017 Aug 8;7(8):e1198. doi: 10.1038/tp.2017.165. Transl Psychiatry. 2017. PMID: 28786980 Free PMC article. - Recent advances in tau-directed immunotherapy against Alzheimer's disease: an overview of pre-clinical and clinical development.
Ng PY, Chang IS, Koh RY, Chye SM. Ng PY, et al. Metab Brain Dis. 2020 Oct;35(7):1049-1066. doi: 10.1007/s11011-020-00591-6. Epub 2020 Jul 6. Metab Brain Dis. 2020. PMID: 32632666 Review.
References
- Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, et al. (2003) Intracranially administered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. The Journal of neuroscience : the official journal of the Society for Neuroscience 23: 3745–3751. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous