A non-cardiomyocyte autonomous mechanism of cardioprotection involving the SLO1 BK channel - PubMed (original) (raw)
A non-cardiomyocyte autonomous mechanism of cardioprotection involving the SLO1 BK channel
Andrew P Wojtovich et al. PeerJ. 2013.
Abstract
Opening of BK-type Ca(2+) activated K(+) channels protects the heart against ischemia-reperfusion (IR) injury. However, the location of BK channels responsible for cardioprotection is debated. Herein we confirmed that openers of the SLO1 BK channel, NS1619 and NS11021, were protective in a mouse perfused heart model of IR injury. As anticipated, deletion of the Slo1 gene blocked this protection. However, in an isolated cardiomyocyte model of IR injury, protection by NS1619 and NS11021 was insensitive to Slo1 deletion. These data suggest that protection in intact hearts occurs by a non-cardiomyocyte autonomous, SLO1-dependent, mechanism. In this regard, an in-situ assay of intrinsic cardiac neuronal function (tachycardic response to nicotine) revealed that NS1619 preserved cardiac neurons following IR injury. Furthermore, blockade of synaptic transmission by hexamethonium suppressed cardioprotection by NS1619 in intact hearts. These results suggest that opening SLO1 protects the heart during IR injury, via a mechanism that involves intrinsic cardiac neurons. Cardiac neuronal ion channels may be useful therapeutic targets for eliciting cardioprotection.
Keywords: Cardiac neurons; Ischemia; Large conductance potassium channel; NS11021; NS1619; Preconditioning; Reperfusion.
Figures
Figure 1. Representative left ventricular pressure traces from perfused hearts.
Traces are shown for wild-type (WT) and _Slo1_−/− hearts, in the presence of vehicle (Ctrl.), NS1619 or NS11021, as per the methods. Traces are compressed on the time (x) axis. The top and bottom boundaries of the black shaded area represent the systolic and diastolic pressures, respectively. The onset of ischemia (I) and reperfusion (R) are indicated by arrows. Scale bars on each trace represent 10 min. (_x_-axis) and 50 mmHg (_y_-axis).
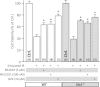
Figure 2. SLO1 dependent protection of perfused mouse heart against ischemia-reperfusion (IR) injury.
Perfused FVB littermate WT (white symbols) and _Slo1_−/− (gray symbols) mouse hearts were subjected to IR injury. Where indicated, hearts were treated with NS1619 (5 µM) or NS11021 (500 nM) prior to ischemia. (A): Left-ventricular function was determined as rate pressure product (RPP; heart rate × left ventricular developed pressure) and expressed as percent of initial value. Statistical significance between groups was determined using a mixed-effects model. *p < 0.05 between NS11021 and control (IR alone), #p < 0.05 between NS1619 and control (IR alone). (B): Following IR protocols, hearts were sliced, stained with TTC and fixed to delineate live (red) from dead or infarcted tissue (white). Infarcts were quantified by planimetry and expressed as a percent of the area at risk (100% in this global ischemia model). Images above the graph show representative slices from each group. Within each group, individual values are on the left, and means ± SEM on the right (N = 7 for IR group and 6 for all other groups). *p < 0.05 between NS11021 and control (IR alone), #p < 0.05 between NS1619 and control (IR alone).
Figure 3. SLO1 independent protection of adult mouse cardiomyocytes against simulated ischemia-reperfusion (IR) injury.
Myocytes were isolated from adult littermate WT (white) and _Slo1_−/− (gray) mouse hearts, and used immediately in the model of IR injury. Where indicated, cells were treated with NS1619 (5 µM), NS11021 (500 nM) or diazoxide (DZX, 10 µM). Upon completion of the IR protocol, cell viability was measured via Trypan blue exclusion and expressed as a percent of control (viability in control normoxic groups was: WT 70 ± 3%, _Slo1_−/− 69 ± 4%). Experimental conditions are listed below the _x_-axis. Data are means ± SEM, with N for each group listed in parentheses at the base off each bar. Each N represents an independent cardiomyocyte preparation. *p < 0.05 vs. IR alone (ANOVA).
Figure 4. SLO1 independent K+ channel activity in isolated mouse heart mitochondria.
Mitochondria were isolated from littermate WT (white) and _Slo1_−/− (gray) mouse hearts and loaded with Tl+-sensitive fluorophore. Mitochondrial K+ channel activity in the presence of NS compounds was determined using the Tl+-flux assay. Data are presented as Δ fluorescence upon Tl+ addition to media. ATP was present to block the mKATP channel. The baseline Δ fluorescence (Ctrl, set to 100%) was 24.7 ± 2.3 and 24.9 ± 4.4 arbitrary units in WT (white bars) and _Slo1_−/− (gray bars), respectively. Experimental conditions are listed below the x_-axis. Data are means ±SEM, with N for each group listed in parentheses at the base off each bar. Each N represents an independent mitochondrial preparation. Control, ATP and ATP + Pax data are replicated between panels A and B for comparison *p < 0.05 vs. ATP, †_p < 0.05 vs. ATP + NS compound (ANOVA).
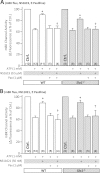
Figure 5. Blocking cardiac neuronal function blocks SLO1 dependent protection in the intact heart.
(A, B): Perfused FVB mouse hearts from WT animals were subjected to IR injury. Where indicated, hearts were treated with NS1619 (5 µM), atpenin A5 (AA5, 10 nM), or hexamethonium (Hex, 500 µM) as detailed in the methods. Left-ventricular function was determined as rate pressure product (RPP; heart rate x left ventricular developed pressure) and expressed as a percent of initial value. Data are split across two panels for clarity. Statistical significance between groups was determined using a mixed-effects model. *p < 0.05 vs. IR alone, #p < 0.05 vs. NS1619 + IR. (C): Following IR protocols, hearts were sliced, stained with TTC and fixed to delineate live (red) from dead or infarcted tissue (white). Infarcts were quantified by planimetry and expressed as a percent of the area at risk (100% in this global ischemia model). Images above the graph show representative slices from each group. Within each group, individual values are on the left, and means ±SEM on the right (N = 8 for IR, 7 for AA5 + IR, and 6 for all other groups). *p < 0.05 vs. IR alone, #p < 0.05 vs. NS1619 + IR.
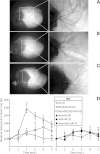
Figure 6. Histochemical staining and functional activity of cardiac neurons.
Perfused FVB mouse hearts from WT animals were subjected to IR injury. Where indicated, hearts were treated with NS1619 (5 µM), atpenin A5 (AA5, 10 nM), hexamethonium (Hex, 500 µM), or combinations of NS1619 + Hex or AA5 + Hex, as detailed in the methods. Panels A–C show representative total heart histochemical acetylcholinesterase (AChE) staining following (A) control perfusion, (B) IR injury and (C) IR with NS1619 pre-treatment. Dorsal side of the heart is shown, with magnifications of boxed areas shown in right panels. (D): Functional activity of cardiac neurons was determined following the six IR protocols used in Fig. 3, via the injection of nicotine (100 µM) for 5 min. The peak heart rate increase was found at 2 min, followed by desensitization and subsequent decrease in heart rate. Data are means ±SEM. N for each group is shown in parentheses to the right of the legend. Each N represents an individual perfused heart. *p < 0.05 vs. IR (ANOVA).
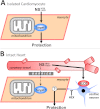
Figure 7. Working model of SLO1 (in)dependent mechanisms of action in NS1619/NS11021 cardioprotection.
(A): In isolated cardiomyocytes the NS compounds induce cardioprotection independent of SLO1. The ‘target’ remains unclear but may include inhibition of respiration or the uncoupling of oxidative phosphorylation (see text for details). This mechanism is compatible with the lack of evidence for SLO1 in cardiomyocytes. (B): In the whole heart, cardioprotection by NS1619/NS11021 is dependent on SLO1 and is likely mediated via a cell non-autonomous mechanism, involving intrinsic cardiac neurons. A barrier is hypothesized to exist, possibly accounting for the inability of NS compounds to recruit the nonspecific target responsible for protection in myocytes. This explains the lack of ability of NS1619/NS11021 to protect the intact _Slo1_−/− heart. HEX = hexamethonium.
Similar articles
- Activation of big conductance Ca(2+)-activated K (+) channels (BK) protects the heart against ischemia-reperfusion injury.
Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M. Bentzen BH, et al. Pflugers Arch. 2009 Mar;457(5):979-88. doi: 10.1007/s00424-008-0583-5. Epub 2008 Sep 2. Pflugers Arch. 2009. PMID: 18762970 - cGMP-Elevating Compounds and Ischemic Conditioning Provide Cardioprotection Against Ischemia and Reperfusion Injury via Cardiomyocyte-Specific BK Channels.
Frankenreiter S, Bednarczyk P, Kniess A, Bork NI, Straubinger J, Koprowski P, Wrzosek A, Mohr E, Logan A, Murphy MP, Gawaz M, Krieg T, Szewczyk A, Nikolaev VO, Ruth P, Lukowski R. Frankenreiter S, et al. Circulation. 2017 Dec 12;136(24):2337-2355. doi: 10.1161/CIRCULATIONAHA.117.028723. Epub 2017 Oct 19. Circulation. 2017. PMID: 29051185 - Pharmacological options to protect the aged heart from ischemia and reperfusion injury by targeting the PKA-BK(Ca) signaling pathway.
Heinen A, Ströthoff M, Schmidt A, Stracke N, Behmenburg F, Bauer I, Hollmann MW, Huhn R. Heinen A, et al. Exp Gerontol. 2014 Aug;56:99-105. doi: 10.1016/j.exger.2014.03.029. Epub 2014 Apr 13. Exp Gerontol. 2014. PMID: 24727217 - Brief structural insight into the allosteric gating mechanism of BK (Slo1) channel 1.
Almássy J, Nánási PP. Almássy J, et al. Can J Physiol Pharmacol. 2019 Jun;97(6):498-502. doi: 10.1139/cjpp-2018-0516. Epub 2018 Dec 5. Can J Physiol Pharmacol. 2019. PMID: 30517027 Review. - The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection.
Smith CO, Nehrke K, Brookes PS. Smith CO, et al. Biochem J. 2017 Jun 9;474(12):2067-2094. doi: 10.1042/BCJ20160623. Biochem J. 2017. PMID: 28600454 Free PMC article. Review.
Cited by
- Inhibition of BKCa channels protects neonatal hearts against myocardial ischemia and reperfusion injury.
Sanghvi S, Szteyn K, Ponnalagu D, Sridharan D, Lam A, Hansra I, Chaudhury A, Majumdar U, Kohut AR, Gururaja Rao S, Khan M, Garg V, Singh H. Sanghvi S, et al. Cell Death Discov. 2022 Apr 7;8(1):175. doi: 10.1038/s41420-022-00980-z. Cell Death Discov. 2022. PMID: 35393410 Free PMC article. - The cardioprotective compound cloxyquin uncouples mitochondria and induces autophagy.
Zhang J, Nadtochiy SM, Urciuoli WR, Brookes PS. Zhang J, et al. Am J Physiol Heart Circ Physiol. 2016 Jan 1;310(1):H29-38. doi: 10.1152/ajpheart.00926.2014. Epub 2015 Oct 30. Am J Physiol Heart Circ Physiol. 2016. PMID: 26519034 Free PMC article. - Inhibition of BKCa negatively alters cardiovascular function.
Patel NH, Johannesen J, Shah K, Goswami SK, Patel NJ, Ponnalagu D, Kohut AR, Singh H. Patel NH, et al. Physiol Rep. 2018 Jun;6(12):e13748. doi: 10.14814/phy2.13748. Physiol Rep. 2018. PMID: 29932499 Free PMC article. - KCNMA1 encoded cardiac BK channels afford protection against ischemia-reperfusion injury.
Soltysinska E, Bentzen BH, Barthmes M, Hattel H, Thrush AB, Harper ME, Qvortrup K, Larsen FJ, Schiffer TA, Losa-Reyna J, Straubinger J, Kniess A, Thomsen MB, Brüggemann A, Fenske S, Biel M, Ruth P, Wahl-Schott C, Boushel RC, Olesen SP, Lukowski R. Soltysinska E, et al. PLoS One. 2014 Jul 29;9(7):e103402. doi: 10.1371/journal.pone.0103402. eCollection 2014. PLoS One. 2014. PMID: 25072914 Free PMC article. - The BKCa (slo) channel regulates the cardiac function of Drosophila.
Gururaja Rao S, Lam A, Seeley S, Park J, Aruva S, Singh H. Gururaja Rao S, et al. Physiol Rep. 2024 Apr;12(7):e15996. doi: 10.14814/phy2.15996. Physiol Rep. 2024. PMID: 38561252 Free PMC article.
References
- Armour JA. Histamine-sensitive intrinsic cardiac and intrathoracic extracardiac neurons influence cardiodynamics. American Journal of Physiology. 1996;270:R906–R913. - PubMed
- Armour JA. Intrinsic cardiac neurons involved in cardiac regulation possess alpha 1-, alpha 2-, beta 1- and beta 2-adrenoceptors. Canadian Journal of Cardiology. 1997;13:277–284. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous