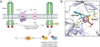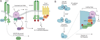Redox regulation of protein kinases - PubMed (original) (raw)
Review
Redox regulation of protein kinases
Thu H Truong et al. Crit Rev Biochem Mol Biol. 2013 Jul-Aug.
Abstract
Protein kinases represent one of the largest families of genes found in eukaryotes. Kinases mediate distinct cellular processes ranging from proliferation, differentiation, survival, and apoptosis. Ligand-mediated activation of receptor kinases can lead to the production of endogenous hydrogen peroxide (H₂O₂) by membrane-bound NADPH oxidases. In turn, H₂O₂ can be utilized as a secondary messenger in signal transduction pathways. This review presents an overview of the molecular mechanisms involved in redox regulation of protein kinases and its effects on signaling cascades. In the first half, we will focus primarily on receptor tyrosine kinases (RTKs), whereas the latter will concentrate on downstream non-receptor kinases involved in relaying stimulant response. Select examples from the literature are used to highlight the functional role of H₂O₂ regarding kinase activity, as well as the components involved in H₂O₂ production and regulation during cellular signaling. In addition, studies demonstrating direct modulation of protein kinases by H₂O₂ through cysteine oxidation will be emphasized. Identification of these redox-sensitive residues may help uncover signaling mechanisms conserved within kinase subfamilies. In some cases, these residues can even be exploited as targets for the development of new therapeutics. Continued efforts in this field will further basic understanding of kinase redox regulation, and delineate the mechanisms involved in physiological and pathological H₂O₂ responses.
Figures
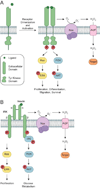
Figure 1
Activation of RTKs and downstream signaling cascades. (a) Growth factors bind to receptor tyrosine kinases (RTKs) to induce receptor dimerization, followed by autophosphorylation of key tyrosine (Tyr) residues (red circles) located within its cytoplasmic domain. In turn, these phosphorylated Tyr residues serve as docking sites for associating proteins to activate a number of downstream signaling cascades. Two such pathways, Ras/ERK and PI3K/AKT, are shown here for simplicity. Ligand-receptor interactions also trigger the assembly and activation of NADPH oxidase (Nox) complexes, followed by subsequent production of H2O2 through spontaneous dismutation or action of superoxide dismutase (SOD). Once formed, endogenous H2O2 may pass through specific aquaporin (AQP) channels and/or diffuse across the membrane to reach the intracellular cytosol. Transient increases in H2O2 lead to the oxidation of localized redox targets. (b) Unlike other RTKs, insulin receptor kinase (IRK) exists as a heterotetrameric receptor composed of two extracellular α subunits and two transmembrane β subunits. Binding of insulin to IRK α subunits induces a conformational change in its quaternary structure to enable ATP binding, receptor autophosphorylation, and production of Nox-derived H2O2. Once activated, IRK recruits members of the insulin receptor substrate (IRS) protein family to initiate glucose metabolism through the PI3K/AKT pathway. Insulin signaling also has mitogenic effects that are mediated through Ras/ERK.
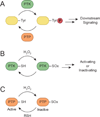
Figure 2
Model for redox-dependent signal transduction. (a) Protein tyrosine kinases (PTKs) catalyze the transfer of γ-phosphoryl groups from ATP to tyrosine hydroxyls of proteins, whereas protein tyrosine phosphatases (PTPs) remove phosphate groups from phosphorylated tyrosine residues. (b) Regulatory cysteines in protein kinases can undergo oxidation/reduction to modulate their function. Depending on the kinase, redox modifications can stimulate or inhibit enzymatic function. (c) PTPs function in a coordinated manner with PTKs to control signaling pathways to regulate a diverse array of cellular processes. Oxidation of the conserved active site cysteine residue in PTPs inactivates these enzymes, and can be restored by reducing the oxidized residue back to its thiol form. SOx: oxidized cysteine.
Figure 3
Oxidative modification of cysteine residues by hydrogen peroxide (H2O2). The initial reaction product of a thiolate with H2O2 yields sulfenic acid (RSOH). This modification, also known as sulfenylation, is reversible and can be directly reduced back to the thiol form or indirectly through disulfide bond formation. Sulfenic acids can be stabilized by the protein microenvironment and/or undergo subsequent modification. For example, sulfenic acids can condense with a second cysteine in the same or different protein to generate disulfide bonds. Alternatively, reaction with the low molecular weight thiol glutathione (GSH, red circle) affords a mixed disulfide through a process known as _S_-glutathionylation. In some proteins, such as PTP1B, nucleophilic attack of a backbone amide on RSOH results in sulfenamide formation. Sulfenyl groups can also oxidize further to the sulfinic (RSO2H) and/or sulfonic (RSO3H) acid forms under conditions of high oxidative stress.
Figure 4
_Trans_-activation of PDGFR and EGFR. Alternative stimulants such as angiotensin II (AngII) can initiate activation of ligand-inaccessible RTKs in lieu of traditional ligand-receptor interactions. In this scenario, AngII binds to GPCRs to promote endogenous H2O2 production and activation of redox regulated PTKs such as c-Src. Src promotes phosphorylation of PDGFR or EGFR, and neighboring receptors can be activated in a lateral-based mechanism. Additionally, concurrent PTP inactivation has also been suggested to promote RTK _trans_-activation.
Figure 5
Isoform-specific roles of peroxiredoxin (Prx) during redox-based PDGFR signaling. (a) PrxII functions as a negative regulator of PDGF signaling. Upon growth factor stimulation, active PrxII is recruited to the membrane and serves to relieve oxidative inactivation of membrane-associated PTPs by eliminating localized H2O2 production within the PDGFR microenvironment. (b) Receptor activation can also induce localized phosphorylation and inactivation of PrxI by PTKs, such as the redox-regulated cytoplasmic Src (c-Src). Deactivation of PrxI reduces the redox-buffering capacity adjacent to the cellular membrane, allowing for transient and localized increases in H2O2 for signal transduction. Additionally, increased H2O2 concentrations can also inactivate Prx2 by oxidation of its catalytic cysteine to sulfinic acid.
Figure 6
General strategy for detecting protein sulfenic acids in cells. (a) Chemoselective reaction between 5,5-dimethyl-1,3-cyclohexanedione (dimedone, 1) and sulfenic acid. (b) Azide and alkyne-functionalized small-molecule probes for trapping and tagging protein sulfenic acids include DAz-2 (2) and DYn-2 (3). (c) Detection of protein sulfenic acids in living cells. Target cells are incubated with cell-permeable probes to trap and tag protein sulfenic acids in situ. After labeling, cell lysates are prepared and tagged proteins are bioorthogonally ligated to biotin or fluorescent reporter tags via click chemistry to enable detection by Western blot or in-gel fluorescence. Alternatively, biotinylated proteins can be enriched for proteomic analysis.
Figure 7
Model for H2O2-dependent regulation of EGFR activation. (a) Binding of EGF to the receptor induces production of H2O2 through Nox2. Nox-derived H2O2 directly modifies EGFR to sulfenic acid at a conserved cysteine residue (Cys797) located in its active site, which enhances the receptor’s intrinsic tyrosine kinase activity. Endogenous H2O2 can also oxidize and deactivate localized PTPs, leading to a net increase in EGFR phosphorylation and activation of downstream signaling cascades. (b) Crystal structure of the EGFR kinase domain (PDB 3GT8) bound to AMP-PNP, a hydrolysis resistant ATP analog, and Mg2+. The yellow dashed lines and accompanying numbers indicate the distance (Å) between the γ-sulfur atom of Cys797 and key substrate functional groups. It is important to note that Cys797 can adopt different rotamers, and sulfenylation of this residue may enhance its ability to participate in electrostatic and hydrogen bonding interactions with its substrate.
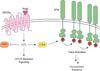
Figure 8
Spatial and temporal modulation of VEGFR2 signaling occurs in discrete subcellular compartments. (a) In the basal state, caveolin-1 (Cav1) negatively modulates VEGFR2 by binding to the receptor in caveolae/lipid rafts. VEGF initiates activation of VEGFR2 by promoting dissociation of Cav1 from the receptor. Once released, activated VEGFR2 localizes with phosphorylated Cav1 and paxillin (Pax) at focal adhesions/complexes to initiate downstream signaling. Growth factor stimulation also recruits small GTPases, ARF6 and Rac1, to activate Nox complexes located in caveolae/lipid rafts. Localized production of H2O2 acts as a secondary messenger during VEGF signaling to promote angiogenesis, proliferation, and migration in endothelial cells (ECs). (b) Endothelial migration is a key event that occurs during angiogenesis in ECs. Cell-cell adhesions are mediated by interactions between VE-cadherin and IQGAP1, which are disrupted upon VEGF stimulation to initiate the migration process. During active migration, IQGAP1 functions as a scaffolding protein to recruit signaling components such as activated VEGFR2, Nox, and Rac1 to the leading edge. Additionally, Nox-derived H2O2 induces localized sulfenic acid formation in IQGAP1 to promote directional migration events. Adapted from Ushio-Fukai, 2007.
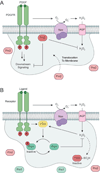
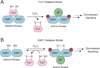
Figure 9
Two proposed models for redox-based activation of ASK1. (a) Trx1 oxidation model. In the cell, ASK1 assembles into multimers that interact with Trx1. Association of Trx1 with ASK1 sequesters the kinase in an inactive conformation that is released upon oxidation of Trx1 by H2O2. Once released, activated ASK1 interacts with additional binding proteins (BPs) to initiate downstream signaling. (b) ASK1 oxidation model. In this second model, H2O2 induces intermolecular disulfide bond formation between ASK1 monomers to promote kinase activation and multimerization. Trx1 is suggested to negatively regulate ASK1 signaling by maintaining the kinase in its reduced state.
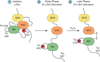
Figure 10
Regulation of c-Src. Inactive c-Src exhibits a “closed” conformation, characterized by binding of phosphorylated Tyr527 to its SH2 domain and interactions between its SH2/SH3 domains. Growth factors and cytokines initiate c-Src activation by promoting concurrent dephosphorylation of Tyr527 and disruption of SH2/SH3 interactions to induce an “open” conformation for Tyr416 autophosphorylation. In the late phase of activation, signal-derived H2O2 mediates the formation of an intramolecular disulfide bond between c-Src Cys245 and Cys487. Bond formation promotes kinase activation, Src-mediated cell adhesion, and cytoskeletal reorganization events. Adapted from Giannoni et al., 2010.
Figure 11
IKK activation of NF-κB. Stimulation with cytokines (i.e. TNFα or interleukins) triggers Ser phosphorylation of the IKK complex by upstream kinases such as MEKK1 or Akt. Once activated, IKK phosphorylates IκB proteins, which negatively regulate NF-κB and maintain the latent transcription factor in the cytoplasm. Phosphorylation of IκB unmasks the nuclear localization signal of NF-κB, and promotes nuclear translocation. Once NF-κB is released, IκB proteins are subsequently degraded by proteasomes. In addition, IKK can be inactivated by endogenously produced H2O2 or NO through direct modulation of Cys179.
Figure 12
Abbreviated sequence alignment of EGFR and nine additional kinases that harbor a cysteine residue structurally homologous to EGFR Cys797. This group includes two erbB family members, HER2 and HER4.
Similar articles
- Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation.
Truong TH, Carroll KS. Truong TH, et al. Biochemistry. 2012 Dec 18;51(50):9954-65. doi: 10.1021/bi301441e. Epub 2012 Dec 5. Biochemistry. 2012. PMID: 23186290 Free PMC article. Review. - Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology.
Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M. Griendling KK, et al. Arterioscler Thromb Vasc Biol. 2000 Oct;20(10):2175-83. doi: 10.1161/01.atv.20.10.2175. Arterioscler Thromb Vasc Biol. 2000. PMID: 11031201 Review. - Redox regulation of protein kinases as a modulator of vascular function.
Knock GA, Ward JP. Knock GA, et al. Antioxid Redox Signal. 2011 Sep 15;15(6):1531-47. doi: 10.1089/ars.2010.3614. Epub 2011 Mar 31. Antioxid Redox Signal. 2011. PMID: 20849377 Review. - The NADPH Oxidases DUOX1 and NOX2 Play Distinct Roles in Redox Regulation of Epidermal Growth Factor Receptor Signaling.
Heppner DE, Hristova M, Dustin CM, Danyal K, Habibovic A, van der Vliet A. Heppner DE, et al. J Biol Chem. 2016 Oct 28;291(44):23282-23293. doi: 10.1074/jbc.M116.749028. Epub 2016 Sep 20. J Biol Chem. 2016. PMID: 27650496 Free PMC article. - Competition between superoxide and hydrogen peroxide signaling in heterolytic enzymatic processes.
Afanas'ev IB. Afanas'ev IB. Med Hypotheses. 2006;66(6):1125-8. doi: 10.1016/j.mehy.2005.11.046. Epub 2006 Feb 24. Med Hypotheses. 2006. PMID: 16500034
Cited by
- Oxygen in human health from life to death--An approach to teaching redox biology and signaling to graduate and medical students.
Briehl MM. Briehl MM. Redox Biol. 2015 Aug;5:124-139. doi: 10.1016/j.redox.2015.04.002. Epub 2015 Apr 11. Redox Biol. 2015. PMID: 25912168 Free PMC article. Review. - ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases.
Davalli P, Mitic T, Caporali A, Lauriola A, D'Arca D. Davalli P, et al. Oxid Med Cell Longev. 2016;2016:3565127. doi: 10.1155/2016/3565127. Epub 2016 May 10. Oxid Med Cell Longev. 2016. PMID: 27247702 Free PMC article. Review. - Mitochondria: Much ado about nothing? How dangerous is reactive oxygen species production?
Holzerová E, Prokisch H. Holzerová E, et al. Int J Biochem Cell Biol. 2015 Jun;63:16-20. doi: 10.1016/j.biocel.2015.01.021. Epub 2015 Feb 7. Int J Biochem Cell Biol. 2015. PMID: 25666559 Free PMC article. Review. - Molecular Basis for Redox Activation of Epidermal Growth Factor Receptor Kinase.
Truong TH, Ung PM, Palde PB, Paulsen CE, Schlessinger A, Carroll KS. Truong TH, et al. Cell Chem Biol. 2016 Jul 21;23(7):837-848. doi: 10.1016/j.chembiol.2016.05.017. Epub 2016 Jul 14. Cell Chem Biol. 2016. PMID: 27427230 Free PMC article. - Mitochondrial trafficking and redox/phosphorylation signaling supporting cell migration phenotypes.
Shannon N, Gravelle R, Cunniff B. Shannon N, et al. Front Mol Biosci. 2022 Jul 22;9:925755. doi: 10.3389/fmolb.2022.925755. eCollection 2022. Front Mol Biosci. 2022. PMID: 35936783 Free PMC article. Review.
References
- Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem. 1997;272:20389–20394. - PubMed
- Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. - PubMed
- Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–35385. - PubMed
- Akhand AA, Pu M, Senga T, Kato M, Suzuki H, Miyata T, Hamaguchi M, Nakashima I. Nitric oxide controls src kinase activity through a sulfhydryl group modification-mediated Tyr-527-independent and Tyr-416-linked mechanism. J Biol Chem. 1999;274:25821–25826. - PubMed
- Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources