Parathyroid hormone-related protein specifies the mammary mesenchyme and regulates embryonic mammary development - PubMed (original) (raw)
Review
Parathyroid hormone-related protein specifies the mammary mesenchyme and regulates embryonic mammary development
Minoti Hiremath et al. J Mammary Gland Biol Neoplasia. 2013 Jun.
Abstract
Parathyroid Hormone related Protein (PTHrP) is a critical regulator of mammary gland morphogenesis in the mouse embryo. Loss of PTHrP, or its receptor, PTHR1, results in arrested mammary buds at day 15 of embryonic development (E15). In contrast, overexpression of PTHrP converts the ventral epidermis into hairless nipple skin. PTHrP signaling appears to be critical for mammary mesenchyme specification, which in turn maintains mammary epithelial identity, directs bud outgrowth, disrupts the male mammary rudiment and specifies the formation of the nipple. In the embryonic mammary bud, PTHrP exerts its effects on morphogenesis, in part, through epithelial-stromal crosstalk mediated by Wnt and BMP signaling. Recently, PTHLH has been identified as a strong candidate for a novel breast cancer susceptibility locus, although PTHrP's role in breast cancer has not been clearly defined. The effects of PTHrP on the growth of the embryonic mammary rudiment and its invasion into the dermis may, in turn, have connections to the role of PTHrP in breast cancer.
Figures
Figure 1. Expression of PTHrP-lacZ in the mammary placodes
X-gal staining is seen in the mammary placode (A) and in cells of the mammary line (A, arrow). At E12.5, staining is observed in all five placodes (B) and along a “tail” of cells extending outwards from the placodes (arrowheads). Staining is also observed in the cells between the mammary placodes (B, arrows). FL= forelimb bud, HL= hindlimb bud.
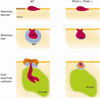
Figure 2. Schematic representation of the consequences of PTHrP/PTHR1 deficiency
Mammary epithelium (pink, ME) is specified in WT, PTHrP−/− and PTHR1−/− embryos. Loss of PTHrP and PTHR1 results in reduced mammary mesenchyme (blue, MM) that does not support outgrowth of the mammary rudiment (MR) or formation of the nipple. Instead, the mammary buds revert to an epidermal fate (reverting bud, RB).
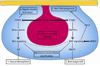
Figure 3. Downstream signaling engaged by PTHrP during embryonic mammary development
PTHrP is secreted from the mammary epithelium (pink) and acts on the mammary mesenchyme (blue) to activate signaling via β-catenin. This pathway directs sexual dimorphism, in part via regulation of AR expression, and possibly regulates the response to BMP signaling. PTHrP signaling, via PTHR1, also upregulates BMPR1a and sensitizes mammary mesenchymal cells to BMP4, possibly upregulating BMP signaling in the mammary mesenchyme. This pathway is required for bud outgrowth, via MMP2 and for inhibiting hair folliculogenesis in the nipple, via Msx2. PTHrP signaling also maintains mammary epithelial identity via signaling in the mammary mesenchyme, although the exact mechanism remains unknown.
References
- Broadus AE, Mangin M, Ikeda K, Insogna KL, Weir EC, Burtis WJ, et al. Humoral hypercalcemia of cancer. Identification of a novel parathyroid hormone-like peptide. N. Engl J Med. 1988;319(9):556–563. - PubMed
- Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996;76(1):127–173. - PubMed
- Jüppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254(5034):1024–1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA153702/CA/NCI NIH HHS/United States
- R01 DK55501/DK/NIDDK NIH HHS/United States
- P20 GM103408/GM/NIGMS NIH HHS/United States
- R01 DK055501/DK/NIDDK NIH HHS/United States
- P20 RR016454/RR/NCRR NIH HHS/United States
- R01 DK077565/DK/NIDDK NIH HHS/United States
- P20GM103408/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials