The protective effect of recombinant FomA-expressing Lactobacillus acidophilus against periodontal infection - PubMed (original) (raw)
The protective effect of recombinant FomA-expressing Lactobacillus acidophilus against periodontal infection
Li Ma et al. Inflammation. 2013 Oct.
Abstract
A number of studies have shown that the outer membrane protein FomA found in Fusobacterium nucleatum demonstrates great potential as an immune target for combating periodontitis. Lactobacillus acidophilus is a useful antigen delivery vehicle for mucosal immunisation, and previous studies by our group have shown that L. acidophilus acts as a protective factor in periodontal health. In this study, making use of the immunogenicity of FomA and the probiotic properties of L. acidophilus, we constructed a recombinant form of L. acidophilus expressing the FomA protein and detected the FomA-specific IgG in the serum and sIgA in the saliva of mice through oral administration with the recombinant strains. When serum containing FomA-specific antibodies was incubated with the F. nucleatum in vitro, the number of Porphyromonas gingivalis cells that coaggregated with the F. nucleatum cells was significantly reduced. Furthermore, a mouse gum abscess model was successfully generated, and the range of gingival abscesses in the immune mice was relatively limited compared with the control group. The level of IL-1β in the serum and local gum tissues of the immune mice was consistently lower than in the control group. Our findings indicated that oral administration of the recombinant L. acidophilus reduced the risk of periodontal infection with P. gingivalis and F. nucleatum.
Figures
Fig. 1
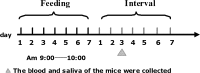
Schematic diagram of the process of feeding with the recombinant L. acidophilus cells. Oral doses (100 μl) were administered daily via gavage between 9 and 10
a.m
. for 7 days, followed by a 7-day interval. Next, a second 7-day cycle of feeding followed by 7 days of withdrawal was performed. Blood and saliva samples were collected once a week.
Fig. 2
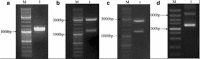
Construction of the recombinant strains and electrophoretic analyses. M represents the DL 10,000 marker (TAKARA, Japan). a Electrophoresis of the PCR products generated using specific fomA primers. 1 The fomA gene (1,046 bp). b Electrophoresis of the PUC57–fomA plasmid digested with _Xba_I and _Hin_dIII. 1 Restriction enzyme fragments, 2,700 and 1,046 bp. c Electrophoresis of the pUC57–fomA–Usp45 plasmid digested with _Xba_I and _Hin_dIII. 1 Restriction enzyme fragments, 2,700 and 1,259 bp. d Electrophoresis of the pMG36e–fomA–Usp45 plasmid digested with _Xba_I and _Hin_dIII. 1 Restriction enzyme fragments, 1,200 and 3,600 bp.
Fig. 3
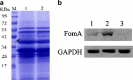
Expression and identification of the fomA protein. a SDS-PAGE analysis showing 41-kDa FomA (arrows) expressed in L. acidophilus (lane 1), the control strain (lane 2), and protein standards (lane M). b Western blot analysis showing that FomA protein was expressed at higher levels in L. acidophilus expressing fomA fused to the Usp45 signal peptide (lane 2) compared with L. acidophilus expressing fomA without Usp45 (lane 1) or the empty vector (lane 3).
Fig. 4
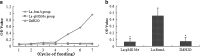
Anti-FomA antibody levels following oral vaccination with L. acidophilus expressing the FomA protein. The difference between the OD 450 and OD 570 nm was taken as the final index. a Levels of FomA-specific IgG in the serum of the immunised mice. b Levels of FomA-specific sIgA in the saliva of the immunised mice. *Compared with the control group, a p value < 0.05 is considered statistically significant.
Fig. 5
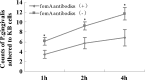
Results of adhesion assays. After P. gingivalis and F. nucleatum were co-cultured with KB cells for 1, 2, or 4 h, the number of P. gingivalis cells adhered to the KB cells, mediated by F. nucleatum, was assessed. The level of P. gingivalis in the experimental group was lower than in the control group at all three time points (p < 0.05).
Fig. 6
Morphologies of swollen gums in mice treated with P. gingivalis plus F. nucleatum. After 3 days of injection, the gums surrounding the lower incisors were red and swollen, and abscesses had formed. The gingival abscesses in the mice in the experimental group were less severe than in the control and blank groups. a The mice immunised with PBS served as the blank group. b The mice immunised with the recombinant strain La-pMG36e served as the control. c The mice immunised with the recombinant strain La-fomA.
Fig. 7
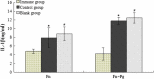
Levels of IL-1β in mouse serum samples. After 3 days of injection, blood was collected from the heart, and the serum was isolated. The levels of IL-1β in the mouse serum were determined using ELISA kits in accordance with the manufacturer's instructions (Thermo Scientific, USA). Following injection with both F. nucleatum alone and F. nucleatum plus P. gingivalis, the mean IL-1β levels in the experimental group of mice were lower than in the control group (*,# p < 0.05).
Fig. 8
Levels of IL-1β in oral mucosal tissues of mice. After 3 days of injection, the mandibular and tongue mucosal tissues of the mice were collected, and 100 mg/ml PBS buffer was added. After homogenisation, the supernatant was collected by centrifugation at 20,000×g at 4 °C for 20 min. The IL-1β levels in the serum and oral mucosal tissues were determined using ELISA kits (Thermo Scientific, USA). The IL-1β levels in the experimental group of mice were lower than in the control group following injections with both F. nucleatum alone and with F. nucleatum plus P. gingivalis (*,# p < 0.05).
Similar articles
- Biochemical characterization of a recombinant Lactobacillus acidophilus strain expressing exogenous FomA protein.
Ma L, Li F, Zhang X, Feng X. Ma L, et al. Arch Oral Biol. 2018 Aug;92:25-31. doi: 10.1016/j.archoralbio.2018.04.016. Epub 2018 Apr 30. Arch Oral Biol. 2018. PMID: 29747062 - [Acquisition and the immunogenicity of the outer membrane FomA protein of Fusobacterium nucleatum].
Ma L, Feng XP, Wu QL, Zhang XY, Zhang X. Ma L, et al. Zhonghua Kou Qiang Yi Xue Za Zhi. 2018 Oct 9;53(10):674-680. doi: 10.3760/cma.j.issn.1002-0098.2018.10.006. Zhonghua Kou Qiang Yi Xue Za Zhi. 2018. PMID: 30392224 Chinese. - Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: Implication for treatment of periodontal infection and halitosis.
Liu PF, Shi W, Zhu W, Smith JW, Hsieh SL, Gallo RL, Huang CM. Liu PF, et al. Vaccine. 2010 Apr 26;28(19):3496-505. doi: 10.1016/j.vaccine.2010.02.047. Epub 2010 Feb 26. Vaccine. 2010. PMID: 20189489 Free PMC article. - Immunological Pathways Triggered by Porphyromonas gingivalis and Fusobacterium nucleatum: Therapeutic Possibilities?
de Andrade KQ, Almeida-da-Silva CLC, Coutinho-Silva R. de Andrade KQ, et al. Mediators Inflamm. 2019 Jun 24;2019:7241312. doi: 10.1155/2019/7241312. eCollection 2019. Mediators Inflamm. 2019. PMID: 31341421 Free PMC article. Review. - Halitosis vaccines targeting FomA, a biofilm-bridging protein of fusobacteria nucleatum.
Liu PF, Huang IF, Shu CW, Huang CM. Liu PF, et al. Curr Mol Med. 2013 Sep;13(8):1358-67. doi: 10.2174/15665240113139990063. Curr Mol Med. 2013. PMID: 23865430 Review.
Cited by
- Probiotic Species in the Management of Periodontal Diseases: An Overview.
Zhang Y, Ding Y, Guo Q. Zhang Y, et al. Front Cell Infect Microbiol. 2022 Mar 25;12:806463. doi: 10.3389/fcimb.2022.806463. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35402306 Free PMC article. Review. - Evaluation of the antibacterial effects and mechanism of Plantaricin 149 from Lactobacillus plantarum NRIC 149 on the peri-implantitis pathogens.
Lin X, Xu J, Shi Z, Xu Y, Fu T, Zhang L, He F. Lin X, et al. Sci Rep. 2021 Oct 25;11(1):21022. doi: 10.1038/s41598-021-00497-y. Sci Rep. 2021. PMID: 34697350 Free PMC article. - Probiotics for periodontal health-Current molecular findings.
Nguyen T, Brody H, Radaic A, Kapila Y. Nguyen T, et al. Periodontol 2000. 2021 Oct;87(1):254-267. doi: 10.1111/prd.12382. Periodontol 2000. 2021. PMID: 34463979 Free PMC article. Review. - Immunization with alkyl hydroperoxide reductase subunit C reduces Fusobacterium nucleatum load in the intestinal tract.
Guo SH, Wang HF, Nian ZG, Wang YD, Zeng QY, Zhang G. Guo SH, et al. Sci Rep. 2017 Sep 5;7(1):10566. doi: 10.1038/s41598-017-11127-x. Sci Rep. 2017. PMID: 28874771 Free PMC article. - Mucosal Vaccination Against Periodontal Disease: Current Status and Opportunities.
Vaernewyck V, Arzi B, Sanders NN, Cox E, Devriendt B. Vaernewyck V, et al. Front Immunol. 2021 Dec 2;12:768397. doi: 10.3389/fimmu.2021.768397. eCollection 2021. Front Immunol. 2021. PMID: 34925337 Free PMC article. Review.
References
- Marcotte H, Koll-Klais P, Hultberg A, Zhao Y, Gmur R, Mandar R, Mikelsaar M, Hammarstrom L. Expression of single-chain antibody against RgpA protease of Porphyromonas gingivalis in Lactobacillus. Journal of Applied Microbiology. 2006;100:256–263. doi: 10.1111/j.1365-2672.2005.02786.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources