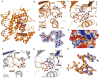Cyclic [G(2',5')pA(3',5')p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase - PubMed (original) (raw)
. 2013 May 23;153(5):1094-107.
doi: 10.1016/j.cell.2013.04.046. Epub 2013 May 3.
Manuel Ascano, Yang Wu, Winfried Barchet, Barbara L Gaffney, Thomas Zillinger, Artem A Serganov, Yizhou Liu, Roger A Jones, Gunther Hartmann, Thomas Tuschl, Dinshaw J Patel
Affiliations
- PMID: 23647843
- PMCID: PMC4382009
- DOI: 10.1016/j.cell.2013.04.046
Cyclic [G(2',5')pA(3',5')p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase
Pu Gao et al. Cell. 2013.
Abstract
Recent studies identified cyclic GMP-AMP (cGAMP) as a metazoan second messenger triggering an interferon response. cGAMP is generated from GTP and ATP by cytoplasmic dsDNA sensor cGAMP synthase (cGAS). We combined structural, chemical, biochemical, and cellular assays to demonstrate that this second messenger contains G(2',5')pA and A(3',5')pG phosphodiester linkages, designated c[G(2',5')pA(3',5')p]. We show that, upon dsDNA binding, cGAS is activated through conformational transitions, resulting in formation of a catalytically competent and accessible nucleotide-binding pocket for generation of c[G(2',5')pA(3',5')p]. We demonstrate that cyclization occurs in a stepwise manner through initial generation of 5'-pppG(2',5')pA prior to cyclization to c[G(2',5')pA(3',5')p], with the latter positioned precisely in the catalytic pocket. Mutants of cGAS dsDNA-binding or catalytic pocket residues exhibit reduced or abrogated activity. Our studies have identified c[G(2',5')pA(3',5')p] as a founding member of a family of metazoan 2',5'-containing cyclic heterodinucleotide second messengers distinct from bacterial 3',5' cyclic dinucleotides.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Figure 1. Structures of cGAMP Synthase in the Free State and Bound to dsDNA
(A) 2.0 Å crystal structure of cGAMP synthase (cGAS) in the free state. The backbone of the protein in a ribbon representation is colored in yellow. (B) 2.1 Å crystal structure of cGAS bound to a complementary 16 bp DNA duplex (with one base 5′ overhang at each end). The protein and DNA are colored in blue in the binary complex. (C) A schematic of intermolecular hydrogen bonds in the binary cGAS-DNA complex. (D) Superposed structures of cGAS in the free state (yellow) and in the cGAS-DNA complex (blue). (E and F) Large changes within the β sheet (E) and catalytic pocket (F) segments were observed for the transition from cGAS in the free state (yellow) to the binary complex with bound DNA (blue). (G) Narrow entrance to the catalytic pocket in the structure of cGAS in the free state, with the protein in an electrostatic representation. (H) Widened entrance to the catalytic pocket in the structure of the binary cGAS-DNA complex. See also Figures S1 and S2 and Table S1.
Figure 2. Structure of the Ternary Complex of cGAS, dsDNA, and ATP
(A) 2.4 Å crystal structure of the ternary complex of cGAS bound to dsDNA and ATP. The protein and dsDNA are colored in salmon in the ternary complex, with bound ATP in a space-filling representation. (B) Superposed structures of the binary complex of cGAS and DNA (blue) and the ternary complex with added ATP (salmon). (C and D) Absence of changes in the backbone within the β sheet (C) and catalytic pocket (D) segments were observed for the transition from the binary cGAS and dsDNA complex (blue) to the ternary complex with added ATP (salmon). (E and F) Two alternate views of intermolecular contacts between ATP and catalytic pocket residues in the ternary complex. Two cations are shown as magenta spheres, with hydrogen bonds shown by dashed red lines. (G) 2Fo-Fc electron density map contoured at 1.2σ (blue) and Fo-Fc map contoured at 3.0σ (red) of bound ATP and a pair of cations and coordinating residues in the catalytic pocket of the ternary complex. This map contains some weak unaccounted for electron density (red). (H) View of bound ATP in a space-filling representation within the catalytic pocket, with the protein in an electrostatic representation. See also Table S2.
Figure 3. Structure of the Ternary Complex of cGAS and dsDNA with Bound 5′-pppG(2′,5′)pG and 5′-pG(2′,5′)pA
(A) 1.9 Å crystal structure of the ternary complex of cGAS bound to dsDNA and 5′-pppG(2′,5′)pG. The protein and DNA are colored in orange in the ternary complex, with bound 5′-pppG(2′,5′)pG in a space-filling representation. (B and C) Two alternate views of intermolecular contacts between 5′-pppG(2′,5′)pG and catalytic pocket residues in the ternary complex. Two cations are shown as magenta spheres, with hydrogen bonds shown by dashed red lines. (D) 2Fo-Fc electron density map contoured at 1.2σ (blue) of bound 5′-pppG(2′,5′)pG in the catalytic pocket of the ternary complex. (E) View of bound 5′-pppG(2′,5′)pG in a space-filling representation within the catalytic pocket, with the protein in an electrostatic representation. (F and G) Two alternate views of intermolecular contacts between 5′-pG(2′,5′)pA and catalytic pocket residues in the 2.3 Å ternary complex of cGAS, dsDNA, and GMP + ATP. (H) Superposition of structures of bound 5′-pppG(2′,5′)pG (orange) and 5′-pG(2′,5′)pA (silver). See also Figure S3 and Tables S2 and S3.
Figure 4. Structure of the Ternary Complex of cGAS and DNA with Bound Product c[G(2′,5′)pA(3′,5′)p]
(A) 2.3 Å crystal structure of the ternary complex of cGAS bound to dsDNA and product c[G(2′,5′)pA(3′,5′)p]. The protein and DNA are colored in green in the ternary complex, with bound product c[G(2′,5′)pA(3′,5′)p] in a space-filling representation. (B and C) Two alternate views of intermolecular contacts between product c[G(2′,5′)pA(3′,5′)p] and catalytic pocket residues in the ternary complex. (D) 2Fo-Fc electron density map contoured at 1.2σ (blue) of bound c[G(2′,5′)pA(3′,5′)p] in the catalytic pocket of the ternary complex. (E) View of bound c[G(2′,5′)pA(3′,5′)p] in a space-filling representation positioned toward one end of the catalytic pocket, with the protein in an electrostatic representation. (F) A view of c[G(2′,5′)pA(3′,5′)p] highlighting the 2′,5′ linkage at the GpA step and the 3′,5′ linkage at the ApG step. (G) Stacking of the G residue of 5′-pG(2′,5′)pA on Tyr 421 in its ternary complex with cGAS and dsDNA. (H) Stacking of the A residue of c[G(2′,5′)pA(3′,5′)p] on Tyr 421 in its ternary complex with cGAS and dsDNA. See also Figure S4 and Table S3.
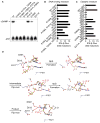
Figure 5. Characterization of c[G(2′,5′) pA(3′,5′)p] Formation by cGAS
Generation of c[G(2′,5′)pA(3′,5′)p] and linear products and intermediates were assayed by thin-layer chromatography (TLC) using purified recombinant truncated (A, amino acids 147–507, used in crystallization studies) and full-length cGAS (B–D, amino acids 1–507). Long- and short-dashed lines indicate the origin and solvent fronts, respectively. (A) A 45 nt dsDNA was incubated with truncated cGAS in reaction buffer containing indicated divalent cation (or EDTA) and α32p-ATP and -GTP. Chemically synthesized cGAMP containing both 3′,5′ linkages was cospotted in every sample, and its migration, visualized by UV, is indicated (dashed outlines). (B) cGAS was incubated with single (ss)-stranded or double (ds)-stranded DNA, RNA, DNA/RNA duplex, or 8-oxoguanine (8-O-G)-modified DNA of similar sequence, and c[G(2′,5′)pA(3′,5′)p] formation was monitored using α32p-ATP. (C) Mono- and diphosphorylated adenosine and guanosine were used as substrates to determine the order of c[G(2′,5′)pA(3′,5′)p] formation. Slow-migrating 2′,5′-linked species was observed when cGAS and dsDNA is incubated with α32p-ATP and GMP (5′-pGpA) or GDP (5′-ppGpA). (D) dsDNA-dependent cGAMP reaction intermediates were visualized by using 2′ or 3′ dATP and dGTP. Slow-migrating intermediate species, corresponding to pppGpA (lane 1) or pppGpdA (lanes 2 and 3), are seen by changing TLC mobile-phase composition. Intermediate species were confirmed using γ32p-GTP. See also Figures S5 and S6 and Table S6.
Figure 6. Definitive Identification of c[G(2′,5′)pA(3′,5′)p] as the Enzymatic Product of cGAS
(A) UV 260 nm chromatographs of GTP, ATP, c[G(2′,5′)pA(2′,5′)p], c[G(3′,5′)pA(3′,5′)p], c[G(2′,5′) pA(3′,5′)p], and cGAS reaction (rxn, asterisk) solutions from reverse-phase HPLC analyses. cGAS reaction samples were injected alone (red) or with addition of indicated reference standards. Shaded region shows the retention time corresponding to the elution of c[G(2′,5′)pA(3′,5′)p]. (B) UV 260 nm chromatographs from HPLC analysis of the cGAS product obtained from dissolved crystals when injected alone (top trace, red) or coinjected with c[G(2′,5′)pA(2′,5′)p] reference compound (middle trace). Additional unidentified peaks were present in the dissolved crystal solution but elute later. The three reference cGAMP compounds were coinjected due to a change (0.5 s) in the retention time of c[G(2′,5′)pA(3′,5′)p] as a result of applying the dissolved crystal solution to the column. (C) NMR spectra of the sugar H1′ proton region of three chemically synthesized cGAMP reference compounds with the cGAS rxn in 99.9% D2O in 10 mM K2HPO4-KH2PO4 (pH 6.6) buffer. The NMR spectrum of the cGAS rxn corresponds to c[G(2′,5′)pA(3′,5′)p] reference compound. The H1′ proton is a doublet (3JHH = 9 Hz) when the phosphate is attached to the 2′ position but a singlet when the phosphate is attached to the 3′ position, reflecting the different puckers of the five-membered sugar ring dependent on the position of the attached phosphate group. See also Figures S7 and S8 and Table S4.
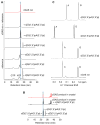
Figure 7. Functional Analysis of cGAS Mutants and Model for Two-Step Generation of c[G(2′,5′)pA(3′,5′)p]
(A) Levels of c[G(2′,5′)pA(3′,5′)p] formation by cGAS full-length WT and indicated mutants were compared by TLC analysis. Long- and short-dashed lines indicate the origin and solvent fronts, respectively. (B and C) Expression vectors of murine cGAS WT or carrying single and multiple alanine mutations of DNA binding (B) and catalytic (C) residues were transiently transfected into HEK293 cells together with an IFN-β Gluc reporter and constitutive STING and Firefly luc expression plasmids. In this setting, expressed cGAS is engaged in the cytosol by the cotransfected DNA plasmids. Gluc values were determined in triplicate 36 hr after transfection, were normalized to Firefly luc, and are shown as fold induction over control plasmid (as mean ± SEM). Data in (B) and (C) are representative of three to five independent experiments for each mutant. (D) A schematic representation of a proposed model associated with a two-step generation of c[G(2′,5′)pA(3′,5′)p] within the single catalytic pocket of cGAS. In this model, the first step involves formation of a 5′-pppGpA intermediate followed by formation of c[G(2′,5′)pA(3′,5′)p]. Note also that the bound ligand is predicted to undergo two flip-overs on the pathway to c[G(2′,5′)pA(3′,5′)p] formation. See also Figure S6 and Table S5.
Comment in
- Building unique bonds to fight misplaced DNA.
Fagundes CT, O'Neill LA. Fagundes CT, et al. Cell Res. 2013 Sep;23(9):1065-6. doi: 10.1038/cr.2013.81. Epub 2013 Jun 18. Cell Res. 2013. PMID: 23774266 Free PMC article.
Similar articles
- The catalytic mechanism of cyclic GMP-AMP synthase (cGAS) and implications for innate immunity and inhibition.
Hall J, Ralph EC, Shanker S, Wang H, Byrnes LJ, Horst R, Wong J, Brault A, Dumlao D, Smith JF, Dakin LA, Schmitt DC, Trujillo J, Vincent F, Griffor M, Aulabaugh AE. Hall J, et al. Protein Sci. 2017 Dec;26(12):2367-2380. doi: 10.1002/pro.3304. Epub 2017 Oct 25. Protein Sci. 2017. PMID: 28940468 Free PMC article. - cGAS-like receptors sense RNA and control 3'2'-cGAMP signalling in Drosophila.
Slavik KM, Morehouse BR, Ragucci AE, Zhou W, Ai X, Chen Y, Li L, Wei Z, Bähre H, König M, Seifert R, Lee ASY, Cai H, Imler JL, Kranzusch PJ. Slavik KM, et al. Nature. 2021 Sep;597(7874):109-113. doi: 10.1038/s41586-021-03743-5. Epub 2021 Jul 14. Nature. 2021. PMID: 34261127 Free PMC article. - Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization.
Li X, Shu C, Yi G, Chaton CT, Shelton CL, Diao J, Zuo X, Kao CC, Herr AB, Li P. Li X, et al. Immunity. 2013 Dec 12;39(6):1019-31. doi: 10.1016/j.immuni.2013.10.019. Immunity. 2013. PMID: 24332030 Free PMC article. - Cyclic GMP-AMP as an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA.
Kato K, Omura H, Ishitani R, Nureki O. Kato K, et al. Annu Rev Biochem. 2017 Jun 20;86:541-566. doi: 10.1146/annurev-biochem-061516-044813. Epub 2017 Apr 7. Annu Rev Biochem. 2017. PMID: 28399655 Review. - Cyclic di-GMP: second messenger extraordinaire.
Jenal U, Reinders A, Lori C. Jenal U, et al. Nat Rev Microbiol. 2017 May;15(5):271-284. doi: 10.1038/nrmicro.2016.190. Epub 2017 Feb 6. Nat Rev Microbiol. 2017. PMID: 28163311 Review.
Cited by
- Multimodal neuro-nanotechnology: Challenging the existing paradigm in glioblastoma therapy.
Kudruk S, Forsyth CM, Dion MZ, Hedlund Orbeck JK, Luo J, Klein RS, Kim AH, Heimberger AB, Mirkin CA, Stegh AH, Artzi N. Kudruk S, et al. Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2306973121. doi: 10.1073/pnas.2306973121. Epub 2024 Feb 12. Proc Natl Acad Sci U S A. 2024. PMID: 38346200 Free PMC article. - STING Targeting in Lung Diseases.
de Moura Rodrigues D, Lacerda-Queiroz N, Couillin I, Riteau N. de Moura Rodrigues D, et al. Cells. 2022 Nov 3;11(21):3483. doi: 10.3390/cells11213483. Cells. 2022. PMID: 36359882 Free PMC article. Review. - Positive feedback regulation of type I IFN production by the IFN-inducible DNA sensor cGAS.
Ma F, Li B, Liu SY, Iyer SS, Yu Y, Wu A, Cheng G. Ma F, et al. J Immunol. 2015 Feb 15;194(4):1545-54. doi: 10.4049/jimmunol.1402066. Epub 2015 Jan 21. J Immunol. 2015. PMID: 25609843 Free PMC article. - The CRL5-SPSB3 ubiquitin ligase targets nuclear cGAS for degradation.
Xu P, Liu Y, Liu C, Guey B, Li L, Melenec P, Ricci J, Ablasser A. Xu P, et al. Nature. 2024 Mar;627(8005):873-879. doi: 10.1038/s41586-024-07112-w. Epub 2024 Feb 28. Nature. 2024. PMID: 38418882 Free PMC article. - PPM1A silences cytosolic RNA sensing and antiviral defense through direct dephosphorylation of MAVS and TBK1.
Xiang W, Zhang Q, Lin X, Wu S, Zhou Y, Meng F, Fan Y, Shen T, Xiao M, Xia Z, Zou J, Feng XH, Xu P. Xiang W, et al. Sci Adv. 2016 Jul 1;2(7):e1501889. doi: 10.1126/sciadv.1501889. eCollection 2016 Jul. Sci Adv. 2016. PMID: 27419230 Free PMC article.
References
- Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases