X-ray structure of an AdoMet radical activase reveals an anaerobic solution for formylglycine posttranslational modification - PubMed (original) (raw)
X-ray structure of an AdoMet radical activase reveals an anaerobic solution for formylglycine posttranslational modification
Peter J Goldman et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Arylsulfatases require a maturating enzyme to perform a co- or posttranslational modification to form a catalytically essential formylglycine (FGly) residue. In organisms that live aerobically, molecular oxygen is used enzymatically to oxidize cysteine to FGly. Under anaerobic conditions, S-adenosylmethionine (AdoMet) radical chemistry is used. Here we present the structures of an anaerobic sulfatase maturating enzyme (anSME), both with and without peptidyl-substrates, at 1.6-1.8 Å resolution. We find that anSMEs differ from their aerobic counterparts in using backbone-based hydrogen-bonding patterns to interact with their peptidyl-substrates, leading to decreased sequence specificity. These anSME structures from Clostridium perfringens are also the first of an AdoMet radical enzyme that performs dehydrogenase chemistry. Together with accompanying mutagenesis data, a mechanistic proposal is put forth for how AdoMet radical chemistry is coopted to perform a dehydrogenation reaction. In the oxidation of cysteine or serine to FGly by anSME, we identify D277 and an auxiliary [4Fe-4S] cluster as the likely acceptor of the final proton and electron, respectively. D277 and both auxiliary clusters are housed in a cysteine-rich C-terminal domain, termed SPASM domain, that contains homology to ~1,400 other unique AdoMet radical enzymes proposed to use [4Fe-4S] clusters to ligate peptidyl-substrates for subsequent modification. In contrast to this proposal, we find that neither auxiliary cluster in anSME bind substrate, and both are fully ligated by cysteine residues. Instead, our structural data suggest that the placement of these auxiliary clusters creates a conduit for electrons to travel from the buried substrate to the protein surface.
Keywords: iron–sulfur cluster fold; radical SAM dehydrogenase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
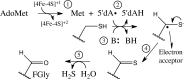
Fig. 1.
anSME reaction. (1) Electron donation to the AdoMet radical cluster initiates homolysis of AdoMet and 5′dA• formation in the presence of bound substrate, (2) substrate radical generation, (3) deprotonation of the substrate Cys sidechain, (4) substrate oxidation, and (5) hydrolysis of the thioaldehyde intermediate yields the FGly moiety of the activated sulfatase.
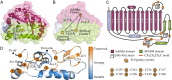
Fig. 2.
Structure of anSMEcpe. (A) The AdoMet domain (magenta) contains the AdoMet cluster and the (β/α)6 partial TIM barrel. The SPASM domain (green) comprises most of the C-terminal segment and houses the remaining two [4Fe-4S] clusters. Two helices, α6a and α6′, are not part of either domain and are colored light blue. (B) Positions and distances between the three [4Fe-4S] clusters (stick representation with Fe in orange and S in yellow). (C) Topology of anSMEcpe. A, AdoMet cluster; I, Aux I; II, Aux II. (D) The SPASM domain, colored by the level of sequence homology between anSMEcpe and the other 280 members of TIGR04085. Conservation scores were calculated by the ConSurf server (40). Iron ligating cysteines are shown as spheres and labeled by their designation in C. Aux I and II are shown in stick representation and labeled.
Fig. 3.
Substrate peptide binding. (A) Cp18Cys (black) and Kp18Cys (gray) enter and exit the active site of anSMEcpe via the underside of the barrel. The Cβ carbon is 8.6 and 8.9 Å from Aux I and the AdoMet cluster, respectively. AdoMet and auxiliary clusters are shown in sticks with carbons in gray, oxygens in red, nitrogens in blue, sulfurs in yellow, and irons in orange. anSMEcpe β strands and SPASM domain are shown in ribbons and colored as in Fig. 2. (B) Substrate peptides bound to anSMEcpe and (C) bound to FGE (PDB ID code 2AIJ, blue) (7) were overlaid by the five residues encompassing the conserved sulfatase motif in each system and are shown in the same orientation.
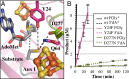
Fig. 4.
anSME active site. (A) The active site of anSMEcpe. Sticks are displayed for AdoMet, target cysteine, and residues within 5 Å of the substrate cysteine Sγ. Distances as follows: AdoMet 5′C–cysteine Cβ, 4.1 Å; Y24–Sγ, 4.7 Å; D277–Sγ, 4.6 Å; Q64 – Sγ, 3.3 Å. Colored as in Fig. 3. (B) FGly and 5′dA production for the Y24F and D277N mutants. Displayed product formation is per μmol enzyme. *Wild-type data from ref. .
Similar articles
- Anaerobic sulfatase-maturating enzyme--a mechanistic link with glycyl radical-activating enzymes?
Benjdia A, Subramanian S, Leprince J, Vaudry H, Johnson MK, Berteau O. Benjdia A, et al. FEBS J. 2010 Apr;277(8):1906-20. doi: 10.1111/j.1742-4658.2010.07613.x. Epub 2010 Mar 9. FEBS J. 2010. PMID: 20218986 Free PMC article. - Mechanistic investigations of anaerobic sulfatase-maturating enzyme: direct Cbeta H-atom abstraction catalyzed by a radical AdoMet enzyme.
Benjdia A, Leprince J, Sandström C, Vaudry H, Berteau O. Benjdia A, et al. J Am Chem Soc. 2009 Jun 24;131(24):8348-9. doi: 10.1021/ja901571p. J Am Chem Soc. 2009. PMID: 19489556 - Anaerobic sulfatase-maturating enzymes, first dual substrate radical S-adenosylmethionine enzymes.
Benjdia A, Subramanian S, Leprince J, Vaudry H, Johnson MK, Berteau O. Benjdia A, et al. J Biol Chem. 2008 Jun 27;283(26):17815-26. doi: 10.1074/jbc.M710074200. Epub 2008 Apr 11. J Biol Chem. 2008. PMID: 18408004 Free PMC article. - SPASM and twitch domains in S-adenosylmethionine (SAM) radical enzymes.
Grell TA, Goldman PJ, Drennan CL. Grell TA, et al. J Biol Chem. 2015 Feb 13;290(7):3964-71. doi: 10.1074/jbc.R114.581249. Epub 2014 Dec 4. J Biol Chem. 2015. PMID: 25477505 Free PMC article. Review. - Structural diversity in the AdoMet radical enzyme superfamily.
Dowling DP, Vey JL, Croft AK, Drennan CL. Dowling DP, et al. Biochim Biophys Acta. 2012 Nov;1824(11):1178-95. doi: 10.1016/j.bbapap.2012.04.006. Epub 2012 Apr 28. Biochim Biophys Acta. 2012. PMID: 22579873 Free PMC article. Review.
Cited by
- Structural, Biochemical, and Bioinformatic Basis for Identifying Radical SAM Cyclopropyl Synthases.
Lien Y, Lachowicz JC, Mendauletova A, Zizola C, Ngendahimana T, Kostenko A, Eaton SS, Latham JA, Grove TL. Lien Y, et al. ACS Chem Biol. 2024 Feb 16;19(2):370-379. doi: 10.1021/acschembio.3c00583. Epub 2024 Jan 31. ACS Chem Biol. 2024. PMID: 38295270 - The Radical SAM Heme Synthase AhbD from Methanosarcina barkeri Contains Two Auxiliary [4Fe-4S] Clusters.
Fix I, Heidinger L, Friedrich T, Layer G. Fix I, et al. Biomolecules. 2023 Aug 18;13(8):1268. doi: 10.3390/biom13081268. Biomolecules. 2023. PMID: 37627333 Free PMC article. - How a Subfamily of Radical S-Adenosylmethionine Enzymes Became a Mainstay of Ribosomally Synthesized and Post-translationally Modified Peptide Discovery.
Mendauletova A, Kostenko A, Lien Y, Latham J. Mendauletova A, et al. ACS Bio Med Chem Au. 2021 Dec 2;2(1):53-59. doi: 10.1021/acsbiomedchemau.1c00045. eCollection 2022 Feb 16. ACS Bio Med Chem Au. 2021. PMID: 37102180 Free PMC article. Review. - Twenty Years of Radical SAM! The Genesis of the Superfamily.
Booker SJ, Lloyd CT. Booker SJ, et al. ACS Bio Med Chem Au. 2022 Dec 5;2(6):538-547. doi: 10.1021/acsbiomedchemau.2c00078. eCollection 2022 Dec 21. ACS Bio Med Chem Au. 2022. PMID: 37101427 Free PMC article. No abstract available. - Preparation and Application of an Inexpensive α-Formylglycine Building Block Compatible with Fmoc Solid-Phase Peptide Synthesis.
Yates NDJ, Warnes ME, Breetveld R, Spicer CD, Signoret N, Fascione M. Yates NDJ, et al. Org Lett. 2023 Mar 31;25(12):2001-2005. doi: 10.1021/acs.orglett.2c04059. Epub 2023 Jan 20. Org Lett. 2023. PMID: 36662590 Free PMC article.
References
- Schmidt B, Selmer T, Ingendoh A, von Figura K. A novel amino acid modification in sulfatases that is defective in multiple sulfatase deficiency. Cell. 1995;82(2):271–278. - PubMed
- Bojarová P, Williams SJ. Sulfotransferases, sulfatases and formylglycine-generating enzymes: A sulfation fascination. Curr Opin Chem Biol. 2008;12(5):573–581. - PubMed
- Dierks T, et al. Multiple sulfatase deficiency is caused by mutations in the gene encoding the human C(alpha)-formylglycine generating enzyme. Cell. 2003;113(4):435–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 RR012408/RR/NCRR NIH HHS/United States
- P41 RR015301/RR/NCRR NIH HHS/United States
- P41 GM103473/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 GM103268/GM/NIGMS NIH HHS/United States
- RR-15301/RR/NCRR NIH HHS/United States
- GM-63847/GM/NIGMS NIH HHS/United States
- P41GM103473/GM/NIGMS NIH HHS/United States
- R01 GM063847/GM/NIGMS NIH HHS/United States
- GM-103268/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources