Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis - PubMed (original) (raw)
Randomized Controlled Trial
. 2013 May 21;110(21):8708-13.
doi: 10.1073/pnas.1300886110. Epub 2013 May 6.
Annamaria Cattaneo, Ksenia Musaelyan, Patricia A Zunszain, Mark Horowitz, Raffaella Molteni, Alessia Luoni, Francesca Calabrese, Katherine Tansey, Massimo Gennarelli, Sandrine Thuret, Jack Price, Rudolf Uher, Marco A Riva, Carmine M Pariante
Affiliations
- PMID: 23650397
- PMCID: PMC3666742
- DOI: 10.1073/pnas.1300886110
Randomized Controlled Trial
Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis
Christoph Anacker et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Stress and glucocorticoid hormones regulate hippocampal neurogenesis, but the molecular mechanisms mediating these effects are poorly understood. Here we identify the glucocorticoid receptor (GR) target gene, serum- and glucocorticoid-inducible kinase 1 (SGK1), as one such mechanism. Using a human hippocampal progenitor cell line, we found that a small molecule inhibitor for SGK1, GSK650394, counteracted the cortisol-induced reduction in neurogenesis. Moreover, gene expression and pathway analysis showed that inhibition of the neurogenic Hedgehog pathway by cortisol was SGK1-dependent. SGK1 also potentiated and maintained GR activation in the presence of cortisol, and even after cortisol withdrawal, by increasing GR phosphorylation and GR nuclear translocation. Experiments combining the inhibitor for SGK1, GSK650394, with the GR antagonist, RU486, demonstrated that SGK1 was involved in the cortisol-induced reduction in progenitor proliferation both downstream of GR, by regulating relevant target genes, and upstream of GR, by increasing GR function. Corroborating the relevance of these findings in clinical and rodent settings, we also observed a significant increase of SGK1 mRNA in peripheral blood of drug-free depressed patients, as well as in the hippocampus of rats subjected to either unpredictable chronic mild stress or prenatal stress. Our findings identify SGK1 as a mediator for the effects of cortisol on neurogenesis and GR function, with particular relevance to stress and depression.
Keywords: antidepressants; hypothalamus–pituitary–adrenal axis; neuroplasticity; stem cells.
Conflict of interest statement
Conflict of interest statement: J.P. acted as a consultant and received payment from ReNeuron Group within the last 2 y. C.M.P. has received fees as a speaker or as a member of advisory board, as well as research funding, from pharmaceutical companies that commercialize or are developing antidepressants in the last 3 y, such as Lilly, Servier, and Janssen. M.A.R. has received compensation as speaker/consultant for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Servier, and Takeda. P.A.Z. has received speaker fees from Servier.
Figures
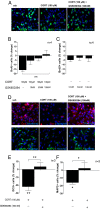
Fig. 1.
CORT (100 µM) decreases neurogenesis via an SGK1-dependent effect. (A) BrdU immunocytochemistry (ICC) was used to assess proliferation. (B) The SGK1 inhibitor, GSK650394 (10 nM, 50 nM, 100 nM) dose-dependently counteracted the CORT-induced reduction in proliferation (n = 4). (C) GSK650394 alone did not change proliferation (n = 4). (D) ICC for doublecortin (Dcx) and microtubule-associated protein 2 (MAP2) was used to assess neuronal differentiation. (E and F) GSK650394 (100 nM) counteracted the CORT-induced decrease in Dcx-positive neuroblasts (n = 3; E) and in MAP2-positive neurons (n = 3; F). Data are mean ± SEM *P < 0.05, **P < 0.01; ***P < 0.001.
Fig. 2.
CORT increases SGK1 expression and inhibits Hedgehog signaling. (A) CORT (100 µM) increases SGK1 mRNA expression after 3, 12, and 72 h (n = 3). (B) CORT (1 nM to 100 µM for 12 h) dose-dependently increases SGK1 mRNA expression (n = 6). (C and D) GSK650394 (100 nM) counteracts the CORT (100 µM)-induced decrease of the Hedgehog pathway genes Gli (n = 6; C) and SMO (n = 6; D). Data are mean ± SEM *P < 0.05, **P < 0.01; ***P < 0.001.
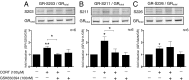
Fig. 3.
SGK1 mediates CORT effects on GR phosphorylation. After 12 h, CORT (100 µM) increases GR phosphorylation at S203 (n = 6; A), S211 (n = 6; B), and S226 (n = 6; C). GSK650394 (100 nM) counteracted the increase in S203 and S211 phosphorylation. Data are mean ± SEM *P < 0.05, **P < 0.01.
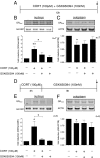
Fig. 4.
SGK1 mediates and maintains CORT effects on GR nuclear translocation. (A) Cotreatment with CORT (100 µM) and GSK650394 (100 nM) for 12 h. (B) GSK650394 counteracted the CORT-induced increase in nuclear GR (n = 7). (C) The CORT-induced decrease in cytoplasmic GR was not counteracted by GSK650394 (n = 7). (D) CORT treatment for the first 3 h and GSK650394 treatment during the subsequent 9 h in the absence of any CORT. (E) CORT (for only 3 h) was sufficient to induce GR nuclear translocation after a total incubation of 12 h. GSK650394 (during the 9-h period after cortisol) abolished the CORT-induced increase in nuclear GR (n = 5). (F) CORT (for only 3 h) decreased cytoplasmic GR. This effect was not counteracted by subsequent GSK650394 treatment (n = 5). Data are mean ± SEM *P < 0.05, **P < 0.01.
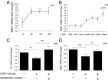
Fig. 5.
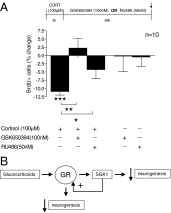
SGK1 effects on proliferation occur upstream and downstream of the GR. (A) CORT (100 µM) for only the first 3 h of a 72-h incubation period was sufficient to reduce the number of BrdU-positive cells (first column). Treatment with GSK650394 (100 nM) during the 69-h period after the initial 3-h CORT stimulus abolished the CORT-induced reduction in proliferation (second column), whereas the GR antagonist RU486 (50 nM) partially counteracted the effect of CORT (third column). n = 10. Data are mean ± SEM *P < 0.05, **P < 0.01; ***P < 0.001. (B) Diagram depicting the proposed model of SGK1-dependent regulation of neurogenesis and GR function.
Fig. 6.
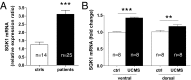
SGK1 mRNA expression is increased in drug-free depressed patients (ctrls, n = 14; patients, n = 25) (A) and the ventral and dorsal hippocampus of rats upon unpredictable chronic mild stress (UCMS, n = 8; B). Data are mean ± SEM; **P < 0.01; ***P < 0.001.
Similar articles
- Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer.
Isikbay M, Otto K, Kregel S, Kach J, Cai Y, Vander Griend DJ, Conzen SD, Szmulewitz RZ. Isikbay M, et al. Horm Cancer. 2014 Apr;5(2):72-89. doi: 10.1007/s12672-014-0173-2. Epub 2014 Mar 11. Horm Cancer. 2014. PMID: 24615402 Free PMC article. - Baicalin promotes hippocampal neurogenesis via SGK1- and FKBP5-mediated glucocorticoid receptor phosphorylation in a neuroendocrine mouse model of anxiety/depression.
Zhang K, Pan X, Wang F, Ma J, Su G, Dong Y, Yang J, Wu C. Zhang K, et al. Sci Rep. 2016 Aug 9;6:30951. doi: 10.1038/srep30951. Sci Rep. 2016. PMID: 27502757 Free PMC article. - Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis.
Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM. Anacker C, et al. Neuropsychopharmacology. 2013 Apr;38(5):872-83. doi: 10.1038/npp.2012.253. Epub 2012 Dec 6. Neuropsychopharmacology. 2013. PMID: 23303060 Free PMC article. - Stress-induced mechanisms in mental illness: A role for glucocorticoid signalling.
Cattaneo A, Riva MA. Cattaneo A, et al. J Steroid Biochem Mol Biol. 2016 Jun;160:169-74. doi: 10.1016/j.jsbmb.2015.07.021. Epub 2015 Aug 1. J Steroid Biochem Mol Biol. 2016. PMID: 26241031 Review. - The glucocorticoid receptor: pivot of depression and of antidepressant treatment?
Anacker C, Zunszain PA, Carvalho LA, Pariante CM. Anacker C, et al. Psychoneuroendocrinology. 2011 Apr;36(3):415-25. doi: 10.1016/j.psyneuen.2010.03.007. Psychoneuroendocrinology. 2011. PMID: 20399565 Free PMC article. Review.
Cited by
- Chronic Corticosterone Administration-Induced Mood Disorders in Laboratory Rodents: Features, Mechanisms, and Research Perspectives.
Wang H, Wang X, Wang H, Shao S, Zhu J. Wang H, et al. Int J Mol Sci. 2024 Oct 19;25(20):11245. doi: 10.3390/ijms252011245. Int J Mol Sci. 2024. PMID: 39457027 Free PMC article. Review. - Sex-specific and developmental effects of early life adversity on stress reactivity are rescued by postnatal knockdown of 5-HT1A autoreceptors.
Dixon R, Malave L, Thompson R, Wu S, Li Y, Sadik N, Anacker C. Dixon R, et al. Neuropsychopharmacology. 2024 Oct 12. doi: 10.1038/s41386-024-01999-9. Online ahead of print. Neuropsychopharmacology. 2024. PMID: 39396089 - Serum/glucocorticoid regulated kinase 1 (SGK1) in neurological disorders: pain or gain.
Howard PG, Zou P, Zhang Y, Huang F, Tesic V, Wu CY, Lee RH. Howard PG, et al. Exp Neurol. 2024 Dec;382:114973. doi: 10.1016/j.expneurol.2024.114973. Epub 2024 Sep 24. Exp Neurol. 2024. PMID: 39326820 Review. - SGK1 suppresses ferroptosis in ovarian cancer via NRF2-dependent and -independent pathways.
Sang X, Han J, Wang Z, Cai W, Liao X, Kong Z, Yu Z, Cheng H, Liu P. Sang X, et al. Oncogene. 2024 Nov;43(45):3335-3347. doi: 10.1038/s41388-024-03173-3. Epub 2024 Sep 21. Oncogene. 2024. PMID: 39306614 - Ketamine Prevents Inflammation-Induced Reduction of Human Hippocampal Neurogenesis via Inhibiting the Production of Neurotoxic Metabolites of the Kynurenine Pathway.
Mandal G, Kirkpatrick M, Alboni S, Mariani N, Pariante CM, Borsini A. Mandal G, et al. Int J Neuropsychopharmacol. 2024 Oct 1;27(10):pyae041. doi: 10.1093/ijnp/pyae041. Int J Neuropsychopharmacol. 2024. PMID: 39297528 Free PMC article.
References
- Mayer JL, et al. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J Neuroendocrinol. 2006;18(8):629–631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases