Serotonin is required for exercise-induced adult hippocampal neurogenesis - PubMed (original) (raw)
Serotonin is required for exercise-induced adult hippocampal neurogenesis
Friederike Klempin et al. J Neurosci. 2013.
Abstract
Voluntary wheel running has long been known to induce precursor cell proliferation in adult hippocampal neurogenesis in rodents. However, mechanisms that couple activity with the promitotic effect are not yet fully understood. Using tryptophan hydroxylase (TPH) 2 deficient (Tph2-deficient) mice that lack brain serotonin, we explored the relationship between serotonin signaling and exercise-induced neurogenesis. Surprisingly, Tph2-deficient mice exhibit normal baseline hippocampal neurogenesis but impaired activity-induced proliferation. Our data demonstrate that the proproliferative effect of running requires the release of central serotonin in young-adult and aged mice. Lack of brain serotonin further results in alterations at the stage of Sox2-positive precursor cells, suggesting physiological adaptations to changes in serotonin supply to maintain homeostasis in the neurogenic niche. We conclude that serotonin plays a direct and acute regulatory role in activity-dependent hippocampal neurogenesis. The understanding of exercise-induced neurogenesis might offer preventive but also therapeutic opportunities in depression and age-related cognitive decline.
Figures
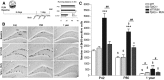
Figure 1.
Lack of TPH2 prevents the proproliferative effect of running. A, The number of proliferating cells in running animals (RUN) and sedentary controls (Baseline) was quantified at day 7, 24 h after the first of three intraperitoneal injections of BrdU. Running distances revealed no differences between genotypes with 1-year-old mice running significant less. B, DAB-staining of BrdU-positive cells in the SGZ for different ages, genotypes, and conditions. GCL, granule cell layer. C, At baseline, the absolute number of proliferating cells is similar between WT and _Tph2_−/− mice at P42 and P80, and is slightly enhanced in 1-year-old _Tph2_−/− mice. Notably, while running robustly increased proliferation in WT animals for all age groups, no effect was seen in P42 and 1-year-old _Tph2_−/− mice with a slight increase at P80. *p < 0.05, **p < 0.01, ***p < 0.001 indicate statistical significance relatively to sedentary controls of the same genotype, #p < 0.05, ##p < 0.01 between WT and _Tph2_−/− for same condition, and $p < 0.05 to previous age (SEM). Scale bar, 120 μm.
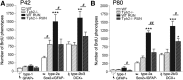
Figure 2.
Running-induced effects on neuronal phenotypes in _Tph2_−/− and WT mice. A, B, The number of BrdU/GFAP quiescent type-1 stem cells did not change between conditions and genotypes at P42 (A) and was slightly altered between WT and _Tph2_−/− after running at P80 (B). Proliferating progenitor cells expressing Sox2 (type 2a) or DCX (type-2b/3) were increased in WT following exercise, and markedly attenuated in _Tph2_−/− mice. Notably, the number of Sox2 type-2a cells was already significantly enhanced in P42 _Tph2_−/− mice at baseline (A). *p < 0.05, **p < 0.01, ***p < 0.001 indicate statistical significance relatively to sedentary controls of the same genotype, and #p < 0.05, ##p < 0.01 between WT and _Tph2_−/− for same condition (SEM).
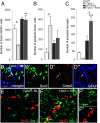
Figure 3.
Sox2-expressing cells are highly affected in _Tph2_−/− mice. A, At P42, the number of overall Sox2/GFAP-expressing cells in _Tph2_−/− mice was significantly lower at baseline but doubled following exercise. B, At baseline, a significantly higher number of Sox2-expressing apoptotic cells (coexpression of Sox2/Caspase3), and increased microgliosis following running (C) was found in _Tph2_−/− mice. D, Confocal microscopy images of Sox2/Caspase3/GFAP coexpression (arrow), and (E) BrdU/iba-1 in _Tph2_−/− mice at P42 baseline levels (BL). F, Increased microgliosis in _Tph2_−/− mice following exercise (arrows indicate coexpression of BrdU/iba-1). Notably, the number of total iba-1-positive microglia is generally increased following exercise independently of genotype, and less frequent in sedentary mice. n.d., not detectable. Scale bar, 25 μm. **p < 0.01, ***p < 0.001 indicate statistical significance relatively to sedentary controls of the same genotype, and #p < 0.05, between WT and _Tph2_−/− for same condition (SEM).
Comment in
- The importance of serotonin in exercise-induced adult neurogenesis: new evidence from Tph2-/- mice.
Beckman D, Santos LE. Beckman D, et al. J Neurosci. 2013 Sep 4;33(36):14283-4. doi: 10.1523/JNEUROSCI.2911-13.2013. J Neurosci. 2013. PMID: 24005280 Free PMC article. No abstract available.
Similar articles
- The effects of brain serotonin deficiency on the behavioral and neurogenesis-promoting effects of voluntary exercise in tryptophan hydroxylase 2 (R439H) knock-in mice.
Warner AK, Iskander L, Allen K, Quatela I, Borrelli H, Sachs BD. Warner AK, et al. Neuropharmacology. 2024 Nov 1;258:110082. doi: 10.1016/j.neuropharm.2024.110082. Epub 2024 Jul 14. Neuropharmacology. 2024. PMID: 39009217 - The importance of serotonin in exercise-induced adult neurogenesis: new evidence from Tph2-/- mice.
Beckman D, Santos LE. Beckman D, et al. J Neurosci. 2013 Sep 4;33(36):14283-4. doi: 10.1523/JNEUROSCI.2911-13.2013. J Neurosci. 2013. PMID: 24005280 Free PMC article. No abstract available. - Involvement of progranulin in the enhancement of hippocampal neurogenesis by voluntary exercise.
Asakura R, Matsuwaki T, Shim JH, Yamanouchi K, Nishihara M. Asakura R, et al. Neuroreport. 2011 Dec 7;22(17):881-6. doi: 10.1097/WNR.0b013e32834bf4ca. Neuroreport. 2011. PMID: 21934540 - Mechanisms underlying the effect of voluntary running on adult hippocampal neurogenesis.
Gao Y, Syed M, Zhao X. Gao Y, et al. Hippocampus. 2023 Apr;33(4):373-390. doi: 10.1002/hipo.23520. Epub 2023 Mar 9. Hippocampus. 2023. PMID: 36892196 Free PMC article. Review. - The role of serotonin in adult hippocampal neurogenesis.
Alenina N, Klempin F. Alenina N, et al. Behav Brain Res. 2015 Jan 15;277:49-57. doi: 10.1016/j.bbr.2014.07.038. Epub 2014 Aug 11. Behav Brain Res. 2015. PMID: 25125239 Review.
Cited by
- Microbiota-Gut-Brain Axis Regulation of Adult Hippocampal Neurogenesis.
Guzzetta KE, Cryan JF, O'Leary OF. Guzzetta KE, et al. Brain Plast. 2022 Oct 21;8(1):97-119. doi: 10.3233/BPL-220141. eCollection 2022. Brain Plast. 2022. PMID: 36448039 Free PMC article. Review. - Adult Neural Stem Cells from Midbrain Periventricular Regions Show Limited Neurogenic Potential after Transplantation into the Hippocampal Neurogenic Niche.
Fauser M, Loewenbrück KF, Rangnick J, Brandt MD, Hermann A, Storch A. Fauser M, et al. Cells. 2021 Nov 4;10(11):3021. doi: 10.3390/cells10113021. Cells. 2021. PMID: 34831242 Free PMC article. - Big brains, meat, tuberculosis, and the nicotinamide switches: co-evolutionary relationships with modern repercussions?
Williams AC, Dunbar RI. Williams AC, et al. Int J Tryptophan Res. 2013 Oct 15;6:73-88. doi: 10.4137/IJTR.S12838. eCollection 2013. Int J Tryptophan Res. 2013. PMID: 24250227 Free PMC article. - Adult Hippocampal Neurogenesis: Regulation and Possible Functional and Clinical Correlates.
Baptista P, Andrade JP. Baptista P, et al. Front Neuroanat. 2018 Jun 5;12:44. doi: 10.3389/fnana.2018.00044. eCollection 2018. Front Neuroanat. 2018. PMID: 29922131 Free PMC article. Review. - On the Run for Hippocampal Plasticity.
Cooper C, Moon HY, van Praag H. Cooper C, et al. Cold Spring Harb Perspect Med. 2018 Apr 2;8(4):a029736. doi: 10.1101/cshperspect.a029736. Cold Spring Harb Perspect Med. 2018. PMID: 28495803 Free PMC article. Review.
References
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boyé P, Vilianovitch L, Sohr R, Tenner K, Hörtnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases