Life without tRNAArg-adenosine deaminase TadA: evolutionary consequences of decoding the four CGN codons as arginine in Mycoplasmas and other Mollicutes - PubMed (original) (raw)
Life without tRNAArg-adenosine deaminase TadA: evolutionary consequences of decoding the four CGN codons as arginine in Mycoplasmas and other Mollicutes
Shin-ichi Yokobori et al. Nucleic Acids Res. 2013 Jul.
Abstract
In most bacteria, two tRNAs decode the four arginine CGN codons. One tRNA harboring a wobble inosine (tRNA(Arg)ICG) reads the CGU, CGC and CGA codons, whereas a second tRNA harboring a wobble cytidine (tRNA(Arg)CCG) reads the remaining CGG codon. The reduced genomes of Mycoplasmas and other Mollicutes lack the gene encoding tRNA(Arg)CCG. This raises the question of how these organisms decode CGG codons. Examination of 36 Mollicute genomes for genes encoding tRNA(Arg) and the TadA enzyme, responsible for wobble inosine formation, suggested an evolutionary scenario where tadA gene mutations first occurred. This allowed the temporary accumulation of non-deaminated tRNA(Arg)ACG, capable of reading all CGN codons. This hypothesis was verified in Mycoplasma capricolum, which contains a small fraction of tRNA(Arg)ACG with a non-deaminated wobble adenosine. Subsets of Mollicutes continued to evolve by losing both the mutated tRNA(Arg)CCG and tadA, and then acquired a new tRNA(Arg)UCG. This permitted further tRNA(Arg)ACG mutations with tRNA(Arg)GCG or its disappearance, leaving a single tRNA(Arg)UCG to decode the four CGN codons. The key point of our model is that the A-to-I deamination activity had to be controlled before the loss of the tadA gene, allowing the stepwise evolution of Mollicutes toward an alternative decoding strategy.
Figures
Figure 1.
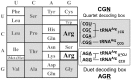
Quartet and duet decoding boxes of the bacterial genetic code, for decoding the 20 amino acids. In the case of arginine, the bacterial tRNAArg set usually involved in decoding Arg codons is also indicated with the respective anticodons.
Figure 2.
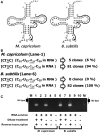
Reverse transcriptase–PCR of tRNAArgICG from M. capricolum and B. subtilis. (A) Comparison of the nucleotide sequences of M. capricolum (Mca) and B. subtilis (Bsu) tRNAArgICG, obtained from (15). The cloverleaf structures are shown. I, 4, D, K, P, 7 and T represent inosine, 4-thio-uridine, dihydrouridine, 1-methylguanosine, pseudouridine, 7-methylguanosine and 5-methyluridine (ribosylthymine), respectively. Regions of primers for reverse transcription of the first strand (and first primers for PCR) are shown with black arrows. Regions of the second primers for PCR are shown with gray arrows. (B) Summary of sequences of cDNA clones for M. capricolum and B. subtilis tRNAArgICG. The DNA sequences of the cDNA clones, except for the PCR primer regions, are shown in brackets. The RNA sequences corresponding to the obtained DNA sequences are shown in parentheses. I (inosine) in the RNA sequence corresponds to G in the DNA sequence obtained by reverse transcription. (C) Agarose gel electrophoresis of reverse transcriptase–PCR products. Lane M: size marker (100-bp ladder, the position of 100 bp is shown with an arrow). Lanes 1–10: PCR products of various templates. Lane 1: reverse-transcribed McatRNAArgICG solution treated with DNase before reverse transcription. Lane 2: total McatRNA solution with DNase treatment. Lane 3: Reverse-transcribed McatRNAArgICG solution without DNase treatment before reverse transcription. Lane 4: total McatRNA solution without DNase treatment. Lane 6: reverse-transcribed BsutRNAArgICG solution with DNase treatment before reverse transcription. Lane 7: total BsutRNA solution with DNase treatment. Lane 8: reverse-transcribed BsutRNAArgICG solution without DNase treatment before reverse transcription. Lane 9: total BsutRNA solution without DNase treatment. Lanes 5 and 10: control (no RNA/DNA).
Figure 3.
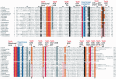
Amino acid sequence alignment of the genes encoding TadA. The TadA amino acid sequences from the species listed in Table 1 were retrieved from Genbank and aligned by Clustal X (34), under the default conditions. The amino acid numbers from E. coli are indicated above the alignment. The amino acid numbers from other species are indicated at the beginning and the end of the sections. The TadA-specific conserved amino acids are highlighted with a red or orange background. The conserved amino acids common among TadA and CDA are highlighted with a black or gray background. The conserved deaminase catalytic and zinc-binding sequences are highlighted in blue or light blue. Structurally and functionally important residues of TadA, inferred from the tertiary structures of the A. aeolicus and S. aureus TadAs (37,44), are indicated above the alignment. The terms ‘nnb’ and ‘stack’ mean non-bonded (hydrophobic) contacts and stacking interactions, respectively. The red boxes in Mollicutes (sequences 2–11) indicate the variations from other bacterial TadAs (sequences 1a–1j). Conserved amino acids involved in tRNA interactions, which are depicted by stick models in Figure 4, are indicated by arrows below the sequences.
Figure 4.
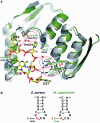
(A) Homology model of M. capricolum TadA, superposed on S. aureus TadA complexed with tRNAArgACG. Both TadA proteins are represented by ribbon models, colored green for M. capricolum and gray for S. aureus. The S. aureus tRNA is depicted by a stick model. Conserved amino acids involved in tRNA interactions, which are indicated by arrows in Figure 3, are shown in stick models. The amino acids specific to Mycoplasma, indicated in the red boxes in Figure 3, are circled. (B) Sequences of the anticodon branches of the tRNAArgACG from S. aureus and M. capricolum (15).
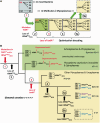
Figure 5.
Hypothetical scenario for the evolution of the CG
N
decoding system for arginine in Mollicutes. (A) Schematic view of the five sequential events leading from a ‘classical bacterial’ arginine decoding strategy involving two tRNAArg, one with a wobble inosine-34 and the other with a wobble C34, to another Arg decoding strategy involving only one tRNAArg with an unmodified wobble U34. In M. capricolum, this latter situation exists in many other quartet decoding boxes (Leu, Val, Ser, Pro, Ala and Gly), as well as in most mitochondria of eukarya. (B) The same events as in A, but depicted within the Mollicute evolutionary framework. Because of the degenerated TadA*, partial A-to-I deamination occurs at the first anticodon position of tRNAArgACG (Step 1), generating a situation where a mixture of both deaminated (in black) and non-deaminated tRNAArg (in red) molecules co-exist in the cell. In addition to the three synonymous arginine codons normally decoded by I34-containing tRNAArgICG, tRNAArgACG also decodes the CG
G
codon, but probably inefficiently (see text). The gene encoding tRNAArgCCG could then be lost (Step 2), along with the gene encoding tad* (Step 3). Further reorganization of the tRNA repertoire could occur by gaining an extra U34-containing tRNAArgUCG (Step 4). The original A34-containing tRNAArgACG can undergo a mutation in its anticodon to generate a G34-containing tRNAArgGCG (Step 5a), or simply be lost (Step 5b). The species of Mollicutes in which these different events occurred are indicated by numbers, corresponding to the organisms listed in Table 1. The phylogenetic relationships among the different Mollicutes were adapted from the literature (29–31).
Similar articles
- Arabidopsis tRNA adenosine deaminase arginine edits the wobble nucleotide of chloroplast tRNAArg(ACG) and is essential for efficient chloroplast translation.
Delannoy E, Le Ret M, Faivre-Nitschke E, Estavillo GM, Bergdoll M, Taylor NL, Pogson BJ, Small I, Imbault P, Gualberto JM. Delannoy E, et al. Plant Cell. 2009 Jul;21(7):2058-71. doi: 10.1105/tpc.109.066654. Epub 2009 Jul 14. Plant Cell. 2009. PMID: 19602623 Free PMC article. - Structure of a tRNA-specific deaminase with compromised deamination activity.
Liu H, Wu S, Ran D, Xie W. Liu H, et al. Biochem J. 2020 Apr 30;477(8):1483-1497. doi: 10.1042/BCJ20190858. Biochem J. 2020. PMID: 32270856 - yaaJ, the tRNA-Specific Adenosine Deaminase, Is Dispensable in Bacillus subtilis.
Soma A, Kubota A, Tomoe D, Ikeuchi Y, Kawamura F, Arimoto H, Shiwa Y, Kanesaki Y, Nanamiya H, Yoshikawa H, Suzuki T, Sekine Y. Soma A, et al. Genes (Basel). 2023 Jul 25;14(8):1515. doi: 10.3390/genes14081515. Genes (Basel). 2023. PMID: 37628567 Free PMC article. - Celebrating wobble decoding: Half a century and still much is new.
Agris PF, Eruysal ER, Narendran A, Väre VYP, Vangaveti S, Ranganathan SV. Agris PF, et al. RNA Biol. 2018;15(4-5):537-553. doi: 10.1080/15476286.2017.1356562. Epub 2017 Sep 21. RNA Biol. 2018. PMID: 28812932 Free PMC article. Review. - ADATs: roles in tRNA editing and relevance to disease.
Mao XL, Eriani G, Zhou XL. Mao XL, et al. Acta Biochim Biophys Sin (Shanghai). 2024 Jul 22;57(1):73-83. doi: 10.3724/abbs.2024125. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 39034823 Free PMC article. Review.
Cited by
- Transfer RNA Modification Enzymes from Thermophiles and Their Modified Nucleosides in tRNA.
Hori H, Kawamura T, Awai T, Ochi A, Yamagami R, Tomikawa C, Hirata A. Hori H, et al. Microorganisms. 2018 Oct 20;6(4):110. doi: 10.3390/microorganisms6040110. Microorganisms. 2018. PMID: 30347855 Free PMC article. Review. - Predicting the minimal translation apparatus: lessons from the reductive evolution of mollicutes.
Grosjean H, Breton M, Sirand-Pugnet P, Tardy F, Thiaucourt F, Citti C, Barré A, Yoshizawa S, Fourmy D, de Crécy-Lagard V, Blanchard A. Grosjean H, et al. PLoS Genet. 2014 May 8;10(5):e1004363. doi: 10.1371/journal.pgen.1004363. eCollection 2014 May. PLoS Genet. 2014. PMID: 24809820 Free PMC article. - Transfer RNA misidentification scrambles sense codon recoding.
Krishnakumar R, Prat L, Aerni HR, Ling J, Merryman C, Glass JI, Rinehart J, Söll D. Krishnakumar R, et al. Chembiochem. 2013 Oct 11;14(15):1967-72. doi: 10.1002/cbic.201300444. Epub 2013 Sep 2. Chembiochem. 2013. PMID: 24000185 Free PMC article. - Life without tRNAIle-lysidine synthetase: translation of the isoleucine codon AUA in Bacillus subtilis lacking the canonical tRNA2Ile.
Köhrer C, Mandal D, Gaston KW, Grosjean H, Limbach PA, Rajbhandary UL. Köhrer C, et al. Nucleic Acids Res. 2014 Feb;42(3):1904-15. doi: 10.1093/nar/gkt1009. Epub 2013 Nov 4. Nucleic Acids Res. 2014. PMID: 24194599 Free PMC article. - Archaeal aminoacyl-tRNA synthetases interact with the ribosome to recycle tRNAs.
Godinic-Mikulcic V, Jaric J, Greber BJ, Franke V, Hodnik V, Anderluh G, Ban N, Weygand-Durasevic I. Godinic-Mikulcic V, et al. Nucleic Acids Res. 2014 Apr;42(8):5191-201. doi: 10.1093/nar/gku164. Epub 2014 Feb 24. Nucleic Acids Res. 2014. PMID: 24569352 Free PMC article.
References
- Agris PF, Vendeix FA, Graham WD. tRNA's wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 2007;366:1–13. - PubMed
- Watanabe K, Suzuki T. Encyclopedia of Life Sciences. Chichester: John Wiley & Sons, Ltd.; 2008. Universal genetic code and its natural variations.
- Grosjean H, de Crécy-Lagard V, Marck C. Deciphering synonymous codons in the three domains of life: co-evolution with specific tRNA modification enzymes. FEBS Lett. 2010;584:252–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials