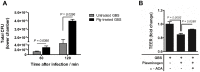Group B Streptococcus hijacks the host plasminogen system to promote brain endothelial cell invasion - PubMed (original) (raw)
Group B Streptococcus hijacks the host plasminogen system to promote brain endothelial cell invasion
Vanessa Magalhães et al. PLoS One. 2013.
Abstract
Group B Streptococcus (GBS) is the leading cause of meningitis in neonates. We have previously shown that plasminogen, once recruited to the GBS cell surface and converted into plasmin by host-derived activators, leads to an enhancement of bacterial virulence. Here, we investigated whether plasmin(ogen) bound at the GBS surface contributes to blood-brain barrier penetration and invasion of the central nervous system. For that purpose, GBS strain NEM316 preincubated with or without plasminogen plus tissue type plasminogen activator was analyzed for the capacity to adhere to, invade and transmigrate the human brain microvascular endothelial cell (hBMEC) monolayer, and to penetrate the central nervous system using a neonatal mouse model. At earlier times of infection, plasmin(ogen)-treated GBS exhibited a significant increase in adherence to and invasion of hBMECs. Later, injury of hBMECs were observed with plasmin(ogen)-treated GBS that displayed a plasmin-like activity. The same results were obtained when hBMECs were incubated with whole human plasma and infected with untreated GBS. To confirm that the observed effects were due to the recruitment and activation of plasminogen on GBS surface, the bacteria were first incubated with epsilon-aminocaproic acid (εACA), an inhibitor of plasminogen binding, and thereafter with plasmin(ogen). A significant decrease in the hBMECs injury that was correlated with a decrease of the GBS surface proteolytic activity was observed. Furthermore, plasmin(ogen)-treated GBS infected more efficiently the brain of neonatal mice than the untreated bacteria, indicating that plasmin(ogen) bound to GBS surface may facilitate the traversal of the blood-brain barrier. A higher survival rate was observed in offspring born from εACA-treated mothers, compared to untreated mice, and no brain infection was detected in these neonates. Our findings suggest that capture of the host plasmin(ogen) by the GBS surface promotes the crossing of the blood-brain barrier and contributes to the establishment of meningitis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
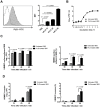
Figure 1. Plasmin(ogen)-coated GBS displays enhanced abilities to adhere to and invade hBMECs in vitro.
(A) GBS cells were incubated with FITC-labeled human plasminogen (hPLG) (grey filled histogram) or PBS (white dotted histogram). Plasminogen binding was measured by a FACScan cytometer as the increase in FITC mean fluorescent intensity (MFI). Each histogram shows cell number as a function of relative fluorescence obtained for 10,000 events per population. Results are shown for 10, 20, and 50 µg of FITC-conjugated hPLG. (B) Representative growth curves of GBS preincubated without (untreated GBS) or with (PLG-treated GBS) plasminogen plus tPA in complete hBMEC growth medium. Data are from a experiment performed in triplicate that is representative of three independent experiments. Each point is the mean of three samples ± SEM. (C and D) HBMEC monolayers were infected with 106 cells of GBS preincubated without (untreated GBS) or with (PLG-treated GBS) plasminogen plus tPA (MOI of 10 bacteria per cell). (C) HBMECs surface adherent GBS cells and (D) intracellular bacteria were isolated and enumerated after 30, 60, and 90 min of infection. The percentages of hBMECs surface adherent GBS and intracellular bacteria are expressed relative to the initial inoculums. Data are the mean + SEM of three independent experiments. Statistical differences (P values) are indicated; ND – not detected.
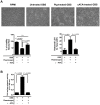
Figure 2. Plasmin(ogen)-coated GBS induces hBMECs detachment and injury.
GBS cells were preincubated without (untreated GBS) or with (PLG-treated GBS) plasminogen plus tPA, or pre-treated with εACA prior to plasminogen plus tPA incubation (εACA-treated GBS). HBMECs monolayers were infected with 106 CFU (MOI of 10) of untreated GBS, PLG-treated GBS or εACA-treated GBS, for 120 min at 37°C; uninfected cells were used as negative controls. (A) Upper panel: representative microscopic photos of the average cell density were taken at ×100 magnification for visualization purposes. Lower left panel: percentage of viable cells, determined by the neutral red assay, expressed relative to the number of viable cells observed in uninfected control. Lower right panel: Cell viability determined by measuring the LDH release. Data represents mean the values normalized to the mean 100%-death control + SEM from an experiment performed in triplicate that is representative of three independent experiments. (B) Plasmin-like activity in bacterial cell surface. The plasmin activity in GBS surface was assessed following incubation with its specific chromogenic substrate S-2251 and determination of the absorbance at 405 nm. Data represents mean + SEM from an experiment performed in triplicate that is representative of three independent experiments.
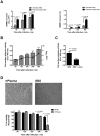
Figure 3. Incubation with human plasma increases the ability of GBS to invade and degrade hBMECs.
(A) HBMECs monolayers were infected with 106 GBS CFU (MOI of 10) pre-incubated or not with whole human plasma and bacterial invasion was determined at the indicated time points and expressed in log 10 CFU/mL (left) or in percentage of intracellular bacteria relative to the initial inoculum. (B) HBMEC monolayer were infected with 106 GBS CFU (MOI of 10) in whole human plasma and the plasmin-like activity of GBS cells was assessed as described in Figure 2C (bars are the mean values of plasmin activity + SEM) and the bacterial CFU were determined at the same time points (line represents the mean numbers of bacterial CFU ± SEM). (C) HBMECs monolayers were infected with 106 GBS CFU (MOI of 10) preincubated (GBS + εACA) or not (GBS) with 200 mM εACA in whole human plasma for a 300 min period at 37°C. The acquisition of cell surface plasmin activity was detected as described in Figure 2C and results are the mean values + SEM of the plasmin activity determined in one experiment performed in triplicate. These data are representative of three independent experiments. Statistical differences (P values) are indicated. (D) Upper panel: representative microscopic photos of the average cell density after 300 min of infection (for visualization purposes, magnification was at 100X). Bottom panel: the percentage of viable cells, assessed by the neutral red assay, was determined as described in Figure 2A. Data are the mean + SEM and are representative of three independent experiments. Statistical differences (P values) are indicated.
Figure 4. Transmigration of GBS across hBMECs.
(A) Confluent hBMECs monolayers grown in the upper chamber of Transwell inserts were infected for a 2 h period with 106 GBS cells preincubated without (untreated GBS) or with (PLG-treated GBS) plasminogen plus tPA. The total lower chamber medium was collected at the indicated time points and total GBS CFU were enumerated. (B) The integrity of the HBMEC monolayers infected with 106 GBS CFU previously incubated with plasminogen plus tPA (PLG-treated GBS), untreated (untreated GBS) or pre-treated with εACA prior to plasminogen plus tPA incubation (εACA-treated GBS) was monitored by measuring the change in TEER. Data are the mean values + SEM of at least two experiments. Statistical differences (P values) are indicated.
Figure 5. Plasmin(ogen)-coated GBS displays enhanced abilities to invade the central nervous system.
(A) Neonatal BALB/c mice were infected i.p. at 48 h after birth with 5×106 CFU of GBS incubated with (PLG-treated GBS) or without (untreated GBS) plasminogen plus tPA. GBS CFU were determined in the brain of neonates at 6 and 18 h post-infection. Results from individual mice are shown. Statistical differences (P values) between groups are indicated. (B and C) Pregnant BALB/c mice, from the gestational day 15 until the end of the experiment, were given drinking water containing εACA (12 g/L) or normal water (control group). The newborns were kept with their mothers throughout the experiments. Two days after the birth, the pups were infected with 5×106 cells of untreated GBS. (B) Kaplan-Meier survival curves of neonatal mice born from εACA-treated or control mothers. The numbers between parentheses represent the number of animals that survive versus the total number of infected animals. Results represent data pooled from two independent experiments. (C) GBS CFU recovered at 18 h post-infection in the liver, lungs, blood and brain of pups. Results from individual mice are shown. Statistical differences (P values) between groups are indicated. ND – not detected.
Similar articles
- Blood-brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin.
Stie J, Fox D. Stie J, et al. Microbiology (Reading). 2012 Jan;158(Pt 1):240-258. doi: 10.1099/mic.0.051524-0. Epub 2011 Oct 13. Microbiology (Reading). 2012. PMID: 21998162 Free PMC article. - Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid.
Doran KS, Engelson EJ, Khosravi A, Maisey HC, Fedtke I, Equils O, Michelsen KS, Arditi M, Peschel A, Nizet V. Doran KS, et al. J Clin Invest. 2005 Sep;115(9):2499-507. doi: 10.1172/JCI23829. J Clin Invest. 2005. PMID: 16138192 Free PMC article. - The role of autophagy during group B Streptococcus infection of blood-brain barrier endothelium.
Cutting AS, Del Rosario Y, Mu R, Rodriguez A, Till A, Subramani S, Gottlieb RA, Doran KS. Cutting AS, et al. J Biol Chem. 2014 Dec 26;289(52):35711-23. doi: 10.1074/jbc.M114.588657. Epub 2014 Nov 4. J Biol Chem. 2014. PMID: 25371213 Free PMC article. - Mechanisms of Blood Brain Barrier Disruption by Different Types of Bacteria, and Bacterial-Host Interactions Facilitate the Bacterial Pathogen Invading the Brain.
Al-Obaidi MMJ, Desa MNM. Al-Obaidi MMJ, et al. Cell Mol Neurobiol. 2018 Oct;38(7):1349-1368. doi: 10.1007/s10571-018-0609-2. Epub 2018 Aug 16. Cell Mol Neurobiol. 2018. PMID: 30117097 Review. - Bacterial plasminogen activators and receptors.
Lähteenmäki K, Kuusela P, Korhonen TK. Lähteenmäki K, et al. FEMS Microbiol Rev. 2001 Dec;25(5):531-52. doi: 10.1111/j.1574-6976.2001.tb00590.x. FEMS Microbiol Rev. 2001. PMID: 11742690 Review.
Cited by
- Group B Streptococcal Neonatal Meningitis.
Tavares T, Pinho L, Bonifácio Andrade E. Tavares T, et al. Clin Microbiol Rev. 2022 Apr 20;35(2):e0007921. doi: 10.1128/cmr.00079-21. Epub 2022 Feb 16. Clin Microbiol Rev. 2022. PMID: 35170986 Free PMC article. Review. - All tangled up: interactions of the fibrinolytic and innate immune systems.
Whyte CS. Whyte CS. Front Med (Lausanne). 2023 Jun 2;10:1212201. doi: 10.3389/fmed.2023.1212201. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37332750 Free PMC article. Review. - Interaction of surface molecules on Cryptococcus neoformans with plasminogen.
Ikeda R, Ichikawa T. Ikeda R, et al. FEMS Yeast Res. 2014 May;14(3):445-50. doi: 10.1111/1567-1364.12131. Epub 2014 Jan 13. FEMS Yeast Res. 2014. PMID: 24373348 Free PMC article. - An opportunistic pathogen under stress: how Group B Streptococcus responds to cytotoxic reactive species and conditions of metal ion imbalance to survive.
Goh KGK, Desai D, Thapa R, Prince D, Acharya D, Sullivan MJ, Ulett GC. Goh KGK, et al. FEMS Microbiol Rev. 2024 May 8;48(3):fuae009. doi: 10.1093/femsre/fuae009. FEMS Microbiol Rev. 2024. PMID: 38678005 Free PMC article. Review. - The plasminogen binding protein PbsP is required for brain invasion by hypervirulent CC17 Group B streptococci.
Lentini G, Midiri A, Firon A, Galbo R, Mancuso G, Biondo C, Mazzon E, Passantino A, Romeo L, Trieu-Cuot P, Teti G, Beninati C. Lentini G, et al. Sci Rep. 2018 Sep 25;8(1):14322. doi: 10.1038/s41598-018-32774-8. Sci Rep. 2018. PMID: 30254272 Free PMC article.
References
- Kim KS (2010) Acute bacterial meningitis in infants and children. Lancet Infect Dis 10: 32–42. - PubMed
- Edwards MS, Rench MA, Haffar AA, Murphy MA, Desmond MM, et al. (1985) Long-term sequelae of group B streptococcal meningitis in infants. J Pediatr 106: 717–722. - PubMed
- Berardi A, Lugli L, Rossi C, China MC, Vellani G, et al. (2010) Neonatal bacterial meningitis. Minerva Pediatr 62: 51–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by research funding from Fundação para a Ciência e Tecnologia (FCT), Fundo Europeu de Desenvolvimento Regional (FEDER) and Programa Operacional Fatores de Competitividade (COMPETE) through the grant n° PTDC/SAU-MIC/111387/2009. Elva Bonifacio Andrade and Joana Alves were supported by a PhD FCT fellowship SFRH/BD/38380/2007 and SRFH/BD/77232/2011, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources