Disease-causing mutations in KLHL3 impair its effect on WNK4 degradation - PubMed (original) (raw)
Disease-causing mutations in KLHL3 impair its effect on WNK4 degradation
Guojin Wu et al. FEBS Lett. 2013.
Abstract
Mutations in with-no-lysine (K) kinase 4 (WNK4) and a ubiquitin E3 ligase complex component kelch-like 3 (KLHL3) both cause pseudohypoaldosteronism II (PHAII), a hereditary form of hypertension. We determined whether WNK4 or its effector is regulated by KLHL3 in Xenopus oocytes. KLHL3 inhibited the positive effect of WNK4 on Na(+)-Cl(-) cotransporter (NCC) by decreasing WNK4 protein abundance without decreasing that of NCC and the downstream kinase OSR1 directly. Ubiquitination and degradation of WNK4 were induced by KLHL3. The effect of KLHL3 on WNK4 degradation was blocked by a dominant negative form of cullin 3. All five PHAII mutations of KLHL3 tested disrupted the regulation on WNK4. We conclude that KLHL3 is a substrate adaptor for WNK4 in a ubiquitin E3 ligase complex.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
Fig. 1
KLHL3 inhibited the effect of WNK4 on NCC-mediated Na+ uptake by decreasing WNK4 protein abundance. (A) KLHL3 inhibited NCC-mediated Na+ uptake and blocked the effect of WNK4 on NCC when expressed in X. laevis oocytes. Data from 33–45 oocytes/group from 4 independent experiments are shown as means ± S.E. * indicates P < 0.05; NS, not significant (_P_ > 0.05). (B) The effects of KLHL3 on the protein abundance of NCC, WNK4, and OSR1. HA-tagged NCC, WNK4, or OSR1 was expressed with HA- or FLAG-tagged KLHL3 in X. laevis oocytes and their protein abundance and that of phosphorylated serine 325 (p-S325) of OSR1 and β-actin (loading control) were assessed by Western blot analysis two days later. The levels of WNK4 and p-S325, but not those of NCC and total OSR1, were reduced by KLHL3.
Fig. 2
The role of CUL3 in WNK4 stability. (A) HA-CUL3 moderately inhibited Na+ uptake of HA-NCC without reducing its protein abundance. Data from 33–35 oocytes/group from 4 independent experiments are shown as means ± S.E. * indicates P < 0.05. (B) Left panel, oocytes were injected with HA-WNK4 cRNA and 12 hrs later, HA-KLHL3 cRNA (or water in the control group) was injected. After 4 hrs, oocytes were treated with protein synthesis blocker cycloheximide (CHX) at 100 µg/ml. Proteins of oocytes were extracted at 12, 16, and 20 hrs after injection of HA-WNK4 cRNA. Upper panel shows the time points of experiment, and lower panel shows the level of HA-WNK4 and HA-KLHL3 at these points. Right panel, co-injection of HA-CUL3 1–400 partially blocked HA-KLHL3 induced reduction in HA-WNK4 abundance. (C) HA-CUL3 1–400 dose-dependently increases the level of HA-WNK4 protein in some batch of oocytes. (D) When the lysate of oocytes expressing GST-CUL3, GST-WNK4, or GST alone was mixed with lysate of oocytes expressing HA-KLHL3, HA-KLHL3 was pulled down by either GST-CUL3 or GST-WNK4 but not by GST alone.
Fig. 3
KLHL3 increased WNK4 ubiquitination. cRNAs for HA-ubiquitin and GST-WNK4 (or GST as control) were injected into oocytes. After 12 hrs, oocytes were injected with cRNA for FLAG-KLHL3 or water as control. GST-WNK4 (A) and GST (B) proteins were pulled down at 0, 3, and 6 hrs after injection of FLAG-KLHL3 or water and the ubiquitinated proteins were detected by HA-antibody (upper panel). The levels of GST-WNK4 (or GST) in pulled down samples and FLAG-KLHL3 in oocytes lysate were detected with GST antibody and FLAG antibody, respectively.
Fig. 4
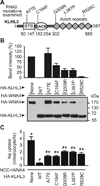
PHAII-causing mutants of KLHL3 were less effective in reducing WNK4 protein abundance in X. laevis oocytes. (A) Representative PHAII-causing mutations examined in this study. (B) HA-WNK4 protein abundance assessed by Western blot analysis in the presence or absence of wild-type (WT) or mutants of HA-KLHL3. HA antibody detected both HA-WNK4 and HA-KLHL3 at different molecular weights. A summary of the band intensity of HA-WNK4 from three experiments is shown in the upper panel. (C) NCC-mediated Na+ uptake in the presence of WNK4 and WT or mutant HA-KLHL3. Similar to the Western blot experiments in (B), uptake experiments were performed at 36 hrs after cRNA injection. Data from 18 oocytes/group derived from 2 frogs are shown as means ± S.E. * indicates P < 0.05 vs. WT HA-KLHL3 group; # indicates P < 0.05 vs. the group without HA-KLHL3.
Similar articles
- The CUL3-KLHL3 E3 ligase complex mutated in Gordon's hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction.
Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. Ohta A, et al. Biochem J. 2013 Apr 1;451(1):111-22. doi: 10.1042/BJ20121903. Biochem J. 2013. PMID: 23387299 Free PMC article. - Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4.
Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Shibata S, et al. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7838-43. doi: 10.1073/pnas.1304592110. Epub 2013 Apr 1. Proc Natl Acad Sci U S A. 2013. PMID: 23576762 Free PMC article. - Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice.
Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, Isobe K, Inoue Y, Takeishi K, Takeda N, Sasaki S, Uchida S. Susa K, et al. Hum Mol Genet. 2014 Oct 1;23(19):5052-60. doi: 10.1093/hmg/ddu217. Epub 2014 May 12. Hum Mol Genet. 2014. PMID: 24821705 - Kelch-like 3/Cullin 3 ubiquitin ligase complex and WNK signaling in salt-sensitive hypertension and electrolyte disorder.
Sohara E, Uchida S. Sohara E, et al. Nephrol Dial Transplant. 2016 Sep;31(9):1417-24. doi: 10.1093/ndt/gfv259. Epub 2015 Jul 6. Nephrol Dial Transplant. 2016. PMID: 26152401 Review. - Regulation of blood pressure and renal electrolyte balance by Cullin-RING ligases.
Uchida S. Uchida S. Curr Opin Nephrol Hypertens. 2014 Sep;23(5):487-93. doi: 10.1097/MNH.0000000000000049. Curr Opin Nephrol Hypertens. 2014. PMID: 24992566 Review.
Cited by
- Unveiling the Distinct Mechanisms by which Disease-Causing Mutations in the Kelch Domain of KLHL3 Disrupt the Interaction with the Acidic Motif of WNK4 through Molecular Dynamics Simulation.
Wang L, Jiang C, Cai R, Chen XZ, Peng JB. Wang L, et al. Biochemistry. 2019 Apr 23;58(16):2105-2115. doi: 10.1021/acs.biochem.9b00066. Epub 2019 Apr 10. Biochemistry. 2019. PMID: 30931564 Free PMC article. - Dual gain and loss of cullin 3 function mediates familial hyperkalemic hypertension.
Cornelius RJ, Zhang C, Erspamer KJ, Agbor LN, Sigmund CD, Singer JD, Yang CL, Ellison DH. Cornelius RJ, et al. Am J Physiol Renal Physiol. 2018 Oct 1;315(4):F1006-F1018. doi: 10.1152/ajprenal.00602.2017. Epub 2018 Jun 13. Am J Physiol Renal Physiol. 2018. PMID: 29897280 Free PMC article. - Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia.
Schumacher FR, Siew K, Zhang J, Johnson C, Wood N, Cleary SE, Al Maskari RS, Ferryman JT, Hardege I, Yasmin, Figg NL, Enchev R, Knebel A, O'Shaughnessy KM, Kurz T. Schumacher FR, et al. EMBO Mol Med. 2015 Oct;7(10):1285-306. doi: 10.15252/emmm.201505444. EMBO Mol Med. 2015. PMID: 26286618 Free PMC article. - Hypertension: the missing WNKs.
Dbouk HA, Huang CL, Cobb MH. Dbouk HA, et al. Am J Physiol Renal Physiol. 2016 Jul 1;311(1):F16-27. doi: 10.1152/ajprenal.00358.2015. Epub 2016 Mar 23. Am J Physiol Renal Physiol. 2016. PMID: 27009339 Free PMC article. Review. - Role of the Ubiquitin Proteasome System in the Regulation of Blood Pressure: A Review.
Yamazaki O, Hirohama D, Ishizawa K, Shibata S. Yamazaki O, et al. Int J Mol Sci. 2020 Jul 28;21(15):5358. doi: 10.3390/ijms21155358. Int J Mol Sci. 2020. PMID: 32731518 Free PMC article. Review.
References
- Paver WK, Pauline GJ. Hypertension and hyperpotassaemia without renal disease in a young male. Med. J. Aust. 1964;2:305–306. - PubMed
- Gordon RD, Geddes RA, Pawsey CG, O'Halloran MW. Hypertension and severe hyperkalaemia associated with suppression of renin and aldosterone and completely reversed by dietary sodium restriction. Australas. Ann. Med. 1970;19:287–294. - PubMed
- Wilson FH, Disse-Nicodeme S, Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases