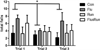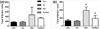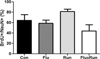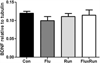Prolonged running, not fluoxetine treatment, increases neurogenesis, but does not alter neuropathology, in the 3xTg mouse model of Alzheimer's disease - PubMed (original) (raw)
Randomized Controlled Trial
Prolonged running, not fluoxetine treatment, increases neurogenesis, but does not alter neuropathology, in the 3xTg mouse model of Alzheimer's disease
Michael W Marlatt et al. Curr Top Behav Neurosci. 2013.
Abstract
Reductions in adult neurogenesis have been documented in the original 3xTg mouse model of Alzheimer's disease (AD), notably occurring at the same age when spatial memory deficits and amyloid plaque pathology appeared. As this suggested reduced neurogenesis was associated with behavioral deficits, we tested whether activity and pharmacological stimulation could prevent memory deficits and modify neurogenesis and/or neuropathology in the 3xTg model backcrossed to the C57Bl/6 strain. We chronically administered the antidepressant fluoxetine to one group of mice, allowed access to a running wheel in another, and combined both treatments in a third cohort. All treatments lasted for 11 months. The female 3xTg mice failed to exhibit any deficits in spatial learning and memory as measured in the Morris water maze, indicating that when backcrossed to the C57Bl/6 strain, the 3xTg mice lost the behavioral phenotype that was present in the original 3xTg mouse maintained on a hybrid background. Despite this, the backcrossed 3xTg mice expressed prominent intraneuronal amyloid beta (Aβ) levels in the cortex and amygdala, with lower levels in the CA1 area of the hippocampus. In the combined cohort, fluoxetine treatment interfered with exercise and reduced the total distance run. The extent of Aβ neuropathology, the tau accumulations, or BDNF levels, were not altered by prolonged exercise. Thus, neuropathology was present but not paralleled by spatial memory deficits in the backcrossed 3xTg mouse model of AD. Prolonged exercise for 11 months did improve the long-term survival of newborn neurons generated during middle-age, whereas fluoxetine had no effect. We further review and discuss the relevant literature in this respect.
Figures
Fig. 1
Total falls in the rotarod test at 20 months of age. During a program of constant acceleration on the rotarod rod, 3xTg mice given fluoxetine showed a higher total number of falls when measured across trials. All three trials were conducted in succession on the same day with approximately 1 h between trials (repeated measures ANOVA F(3,54) = 5.82, p < 0.05)
Fig. 2
BrdU + cell survival 1 and 11 months after beginning treatment. a 1 month after treatment with fluoxetine (Flu), exposure to running (Run), or combined therapy (FluxRun), none of the treatment groups showed significant elevations in BrdU+ cell survival compared to controls (Con) (One-way ANOVA F(3, 9) = 0.97, p = 0.44). b 11 months after starting treatment, significant increases in BrdU+ cell survival were seen for both Run and FluxRun groups compared to nonrunning controls. (One-way ANOVA F(3,23) = 5.27, p < 0.01)
Fig. 3
Percentage of BrdU + cells expressing the mature neuronal marker NeuN. None of the treatment groups showed significant differences in the percentage of BrdU + newborn cells expressing NeuN as marker for mature CNS neurons (One-way ANOVA F(3,19) = 2.27, p = 0.11)
Fig. 4
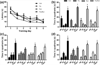
3xTg mice show no impairment in learning or memory during testing in the MWM at 10 or 20 months of age a 1 month data reflects that all groups learned the MWM to criterion (latency to platform < 20 s) (repeated measures ANOVA F(3,198) = 1.97, p = 0.16) and b all groups at the 1 month timepoint, remembered the location of the platform 24 h after their last training session (Con F(3,27) = 13.44, Flu F(3,27) = 54.91, Run F(3,24) = 11.60, FluxRun F(3,27) = 17.09, P < 0.001) Bonferroni pos_t_ test p < 0.05 target versus each quandrant c 10 months after starting the experiment, 3xTg mice were re-trained and Con, Run, and FluxRun showed significant preferences for the target quadrant during 4 h probe trials (Con F(3,12) = 9.00, Run F(3,21) = 6.00, FluxRun F(3,18) = 9.34, P < 0.01) Bonferroni post-test p < 0.05 target versus each quandrant, Flu F(3,21) = 2.94, p = 0.06) d 24 h probe trials (Con F(3,12) = 13.14, Run F(3,21) = 21.50, FluxRun F(3,18) = 22.81, P < 0.01) Bonferroni post-test p < 0.05 target versus each quadrant. Flu did not show a significant preference for the target quadrant
Fig. 5
Mature BDNF protein in the hippocampus. No significant differences were observed in the amount of soluble BDNF across cohorts. BDNF protein, as measured by Western blot, was standardized to the housekeeping gene, beta-tubulin (One-way ANOVA F(3,23) = 0.56, p = 0.64)
Fig. 6
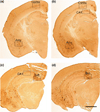
Anatomical overview and regions of interest used for quantification of Aβ pathology in 20 month-old female 3xTg mice. a Cortical and amygdala Aβ pathology were quantified at approximately Bregma −1.46 mm. b Cortical, amygdala and CA1 pathology were quantified at −1.94, while quantification of the subiculum continued through −2.46, c staining of the subiculum was quantified in the caudal hippocampus, here at −2.70 mm, but as far back as −3.30 mm. scale bar = 600 µm
Fig. 7
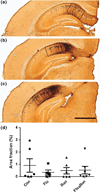
Intraneuronal Aβ and Aβ plaque load in selected subregions of the 3xTg mouse brain. a–d Examples of Aβ deposition in the amygdala of 3xTg mice, showing intraneuronal Aβ and extracellular Aβ deposits across cohorts. e–h Intraneuronal Aβ was found in the cortex of all cohorts, with little evidence of extracellular deposits. i–j In the CA1 region, the majority of detected Aβ was present in extracellular plaques, but no neuronal profiles were seen when compared to the cortex. m–p A dense staining of extracellular plaques was predominantly found in the subiculum across cohorts (scale bar = 100 µm). q Densitometry of the amygdala across cohorts: Flu and FluxRun groups had lower area fractions scores compared to Run, but not to the Con groups. (One-way ANOVA F(3,24) = 4.79, p < 0.05) Bonferroni post-test Flu versus Run p < 0.05, FluxRun versus Run p < 0.05). r In the cortex, fluoxetine appears to reduce Aβ accumulations, however, these differences were not statistically significant (One-way ANOVA F(3,22) = 1.00, p = 0.41)
Fig. 8
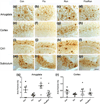
AT8 tau pathology in the CA1 region of the 3xTg mouse. a–c Neurons of the CA1 regions showed robust staining for hyperphorlated tau protein as identified by AT8 immunostaining. While being photographed sections were positioned to exclusively frame CA1. Quantification was carried out on sections beginning at −1.94 mm Bregma and continuing through approximately −3.16 mm Bregma, scale bar = 600 µm. d None of the interventions showed an ability to reduce pathological hyperphosphorylation (ANOVA F(3,22) = 0.58, p = 0.61)
Fig. 9
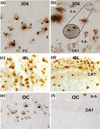
Comparing cortical and hippocampal Aβ accumulations in aged 3xTg mice during backcrossing with three antibodies (3D6, IBL, and OC) In an effort to identify differences due to backcrossing, this figure compares cortical and hippocampal staining in 3xTg mice. a In 20-month-old nonbackcrossed mice, intraneuronal Aβ puncta are condensed in the cortex, increasing density of puncta at the axon terminal is denoted by letters a–d. b This is also seen to a lesser extent in the CA1, where 3D6 labels neuronal somata; diffuse plaques and axonal processes are also seen in the stratum oriens (s.o.) Panels a, b reproduced from Fig. 2 of (Cai et al. 2012) c 20-month-old backcrossed mice, presented in this study, show similar intraneuronal puncta in the cortex with identifiable accumulations at the axon terminals d however, no discernable neuronal profiles are seen in the CA1 of backcrossed 3xTg mice indicating that intraneuronal Aβ is absent in these animals e 18-month-old nonbackcrossed mice are positive for intraneuronal Aβ as detected by conformation specific OC antibody in the cortex. f 3xTg mice on the nonbackcrossed background show low levels of intraneuronal Aβ in the CA1 when measured with conformation specific OC antibody. Panels e, f adapted from Fig. 4 Wirths et al. 2011
Similar articles
- Voluntary Running Attenuates Memory Loss, Decreases Neuropathological Changes and Induces Neurogenesis in a Mouse Model of Alzheimer's Disease.
Tapia-Rojas C, Aranguiz F, Varela-Nallar L, Inestrosa NC. Tapia-Rojas C, et al. Brain Pathol. 2016 Jan;26(1):62-74. doi: 10.1111/bpa.12255. Epub 2015 May 7. Brain Pathol. 2016. PMID: 25763997 Free PMC article. - Protective effect of exercise training against the progression of Alzheimer's disease in 3xTg-AD mice.
Kim D, Cho J, Kang H. Kim D, et al. Behav Brain Res. 2019 Nov 18;374:112105. doi: 10.1016/j.bbr.2019.112105. Epub 2019 Jul 17. Behav Brain Res. 2019. PMID: 31325514 - Ultrasound with microbubbles improves memory, ameliorates pathology and modulates hippocampal proteomic changes in a triple transgenic mouse model of Alzheimer's disease.
Shen Y, Hua L, Yeh CK, Shen L, Ying M, Zhang Z, Liu G, Li S, Chen S, Chen X, Yang X. Shen Y, et al. Theranostics. 2020 Sep 26;10(25):11794-11819. doi: 10.7150/thno.44152. eCollection 2020. Theranostics. 2020. PMID: 33052247 Free PMC article. - Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise.
Micheli L, Ceccarelli M, D'Andrea G, Tirone F. Micheli L, et al. Brain Res Bull. 2018 Oct;143:181-193. doi: 10.1016/j.brainresbull.2018.09.002. Epub 2018 Sep 17. Brain Res Bull. 2018. PMID: 30236533 Review. - Neuroplasticity and behavioral effects of fluoxetine after experimental stroke.
Sun Y, Sun X, Qu H, Zhao S, Xiao T, Zhao C. Sun Y, et al. Restor Neurol Neurosci. 2017;35(5):457-468. doi: 10.3233/RNN-170725. Restor Neurol Neurosci. 2017. PMID: 28854520 Review.
Cited by
- Emerging Therapeutic Potential of Fluoxetine on Cognitive Decline in Alzheimer's Disease: Systematic Review.
Bougea A, Angelopoulou E, Vasilopoulos E, Gourzis P, Papageorgiou S. Bougea A, et al. Int J Mol Sci. 2024 Jun 13;25(12):6542. doi: 10.3390/ijms25126542. Int J Mol Sci. 2024. PMID: 38928248 Free PMC article. Review. - From cradle to grave: neurogenesis, neuroregeneration and neurodegeneration in Alzheimer's and Parkinson's diseases.
Wakhloo D, Oberhauser J, Madira A, Mahajani S. Wakhloo D, et al. Neural Regen Res. 2022 Dec;17(12):2606-2614. doi: 10.4103/1673-5374.336138. Neural Regen Res. 2022. PMID: 35662189 Free PMC article. Review. - Tau Pathology Profile Across a Parietal-Hippocampal Brain Network Is Associated With Spatial Reorientation Learning and Memory Performance in the 3xTg-AD Mouse.
Stimmell AC, Xu Z, Moseley SC, Cushing SD, Fernandez DM, Dang JV, Santos-Molina LF, Anzalone RA, Garcia-Barbon CL, Rodriguez S, Dixon JR, Wu W, Wilber AA. Stimmell AC, et al. Front Aging. 2021 May;2:655015. doi: 10.3389/fragi.2021.655015. Epub 2021 May 19. Front Aging. 2021. PMID: 34746919 Free PMC article. - Identification and drug-induced reversion of molecular signatures of Alzheimer's disease onset and progression in AppNL-G-F, AppNL-F, and 3xTg-AD mouse models.
Pauls E, Bayod S, Mateo L, Alcalde V, Juan-Blanco T, Sánchez-Soto M, Saido TC, Saito T, Berrenguer-Llergo A, Attolini CS, Gay M, de Oliveira E, Duran-Frigola M, Aloy P. Pauls E, et al. Genome Med. 2021 Oct 26;13(1):168. doi: 10.1186/s13073-021-00983-y. Genome Med. 2021. PMID: 34702310 Free PMC article. - Adult neurogenic process in the subventricular zone-olfactory bulb system is regulated by Tau protein under prolonged stress.
Dioli C, Patrício P, Pinto LG, Marie C, Morais M, Vyas S, Bessa JM, Pinto L, Sotiropoulos I. Dioli C, et al. Cell Prolif. 2021 Jul;54(7):e13027. doi: 10.1111/cpr.13027. Epub 2021 May 14. Cell Prolif. 2021. PMID: 33988263 Free PMC article.
References
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–688. - PubMed
- Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006a;24(1):1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous