Decrease of 5-hydroxymethylcytosine is associated with progression of hepatocellular carcinoma through downregulation of TET1 - PubMed (original) (raw)
. 2013 May 9;8(5):e62828.
doi: 10.1371/journal.pone.0062828. Print 2013.
Limei Liu, Xuejiao Chen, Junjie Shen, Juanjuan Shan, Yanmin Xu, Zhi Yang, Lin Wu, Feng Xia, Ping Bie, Youhong Cui, Xiu-wu Bian, Cheng Qian
Affiliations
- PMID: 23671639
- PMCID: PMC3650038
- DOI: 10.1371/journal.pone.0062828
Decrease of 5-hydroxymethylcytosine is associated with progression of hepatocellular carcinoma through downregulation of TET1
Chungang Liu et al. PLoS One. 2013.
Abstract
DNA methylation is an important epigenetic modification and is frequently altered in cancer. Convert of 5-methylcytosine (5 mC) to 5-hydroxymethylcytosine (5 hmC) by ten-eleven translocation (TET) family enzymes plays important biological functions in embryonic stem cells, development, aging and disease. Recent reports showed that level of 5 hmC was altered in various types of cancers. However, the change of 5 hmC level in hepatocellular carcinoma (HCC) and association with clinical outcome were not well defined. Here, we reported that level of 5 hmC was decreased in HCC tissues, as compared with non-tumor tissues. Clincopathological analysis showed the decreased level of 5 hmC in HCC was associated with tumor size, AFP level and poor overall survival. We also found that the decreased level of 5 hmC in non-tumor tissues was associated with tumor recurrence in the first year after surgical resection. In an animal model with carcinogen DEN-induced HCC, we found that the level of 5 hmC was gradually decreased in the livers during the period of induction. There was further reduction of 5 hmC in tumor tissues when tumors were developed. In contrast, level of 5 mC was increased in HCC tissues and the increased 5 mC level was associated with capsular invasion, vascular thrombosis, tumor recurrence and overall survival. Furthermore, our data showed that expression of TET1, but not TET2 and TET3, was downregulated in HCC. Taken together, our data indicated 5 hmC may be served as a prognostic marker for HCC and the decreased expression of TET1 is likely one of the mechanisms underlying 5 hmC loss in HCC.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
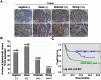
Figure 1. The level of 5 hmC was decreased in human HCC.
(A) Detection of 5 hmC in HCC cells (Huh7 and PLC/PRF/5) by immunofluorescent staining. The representative photomicrographs were presented. (B) Detection of 5 hmC in the paraffin-embedded formalin–fixed HCC tissues by immunohistochemistry. Representative photomicrographs were presented. (C and D) The level of 5 hmC was examined in a tissuearray containing 146 paraffin-embedded formalin–fixed HCC tissues and paired non-HCC counterparts by immunohistochemistry. The representative photomicrographs of 5 hmC level with high, equal and low in HCC tumors, as compared with non-tumor tissues, were shown (C). The altered level of 5 hmC between HCC and non-tumor tissues in 146 HCC patients were summarized (D) (n = number of cases).
Figure 2. The level of 5 hmC in tumor tissues was associated with overall survival in HCC.
(A and B) The level of 5 hmC was analyzed in HCC tissues by immunohistochemistry. Representative photomicrographs showed negative (–), weak (+), moderate (++) and strong (+++) staining in HCC tissues (A). Various levels of 5 hmC in 146 HCC patients were summarized (n = number of cases) (B). (C) Kaplan-Meier curve for overall survival was compared according to the 5 hmC level in HCC tissues.
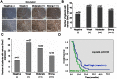
Figure 3. The level of 5 hmC in non-tumor tissues was associated with HCC recurrence in the first year.
(A and B) Level of 5 hmC was analyzed in non-tumor tissues by immunohistochemistry. Representative photomicrographs showed negative (–), weak (+), moderate (++) and strong (+++) staining in HCC non-tumor tissues (A). Various levels of 5 hmC in 146 HCC patients were summarized (n = number of cases) (B). (C) Various levels of 5 hmC in 75 cases of HCC patients with recurrence at the first year were summarized (n = number of cases). (D) Kaplan-Meier curve for overall survival was compared according to 5 hmC expression in non-tumor tissues.
Figure 4. Level of 5 hmC is dynamic changed in an animal model of DEN-induced liver cancer.
DEN was given to rats for 24 weeks for induction of liver cancer. Before induction and different time points after induction, animals were sacrificed and liver tissue samples were collected. (A) Level of 5 hmC was analyzed in the paraffin-embedded formalin–fixed rat liver and tumor tissues by immunohistochemistry. Representative photomicrographs for 5 hmC in liver and tumor tissues were presented. (B) Quantification of 5 hmC level was carried out in liver tissues from normal animals, animals during induction and tumor tissues. (n = 5 animals per group).
Figure 5. The level of 5 mC was increased in human HCC and the increased level of 5 mC was associated with poor prognosis.
(A) Level of 5 mC was analyzed in a tissuearray containing 145 paraffin-embedded formalin–fixed HCC tissues and paired non-HCC counterparts by IHC. Representative photomicrographs showed weak (+), moderate (++) and strong (+++) staining in HCC tissues. (B) The representative photomicrographs of 5 mC level with high, equal and low in HCC tumors, as compared with non-tumor tissues, were shown. (C) The altered level of 5 mC between HCC and non-tumor tissues in 145 HCC patients were summarized (n = number of cases). (D) Kaplan-Meier curve for overall survival was compared according to the 5 mC level in HCC tissues.
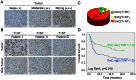
Figure 6. Expression of TET (1/2/3) in HCC and non-tumor tissues.
(A) The protein levels of TET1/2/3 were examined in 20 HCC fresh tumor tissues and paired non-tumor tissues by Western blotting analysis. (B) For quantification, signals were densitometrically normalized to GAPDH. The levels of TET1/2/3 were presented as the mean±SD.
Similar articles
- TET family proteins and 5-hydroxymethylcytosine in esophageal squamous cell carcinoma.
Murata A, Baba Y, Ishimoto T, Miyake K, Kosumi K, Harada K, Kurashige J, Iwagami S, Sakamoto Y, Miyamoto Y, Yoshida N, Yamamoto M, Oda S, Watanabe M, Nakao M, Baba H. Murata A, et al. Oncotarget. 2015 Sep 15;6(27):23372-82. doi: 10.18632/oncotarget.4281. Oncotarget. 2015. PMID: 26093090 Free PMC article. - Role of ten-eleven translocation proteins and 5-hydroxymethylcytosine in hepatocellular carcinoma.
Wang P, Yan Y, Yu W, Zhang H. Wang P, et al. Cell Prolif. 2019 Jul;52(4):e12626. doi: 10.1111/cpr.12626. Epub 2019 Apr 29. Cell Prolif. 2019. PMID: 31033072 Free PMC article. Review. - Reduction of global 5-hydroxymethylcytosine is a poor prognostic factor in breast cancer patients, especially for an ER/PR-negative subtype.
Tsai KW, Li GC, Chen CH, Yeh MH, Huang JS, Tseng HH, Fu TY, Liou HH, Pan HW, Huang SF, Chen CC, Chang HY, Ger LP, Chang HT. Tsai KW, et al. Breast Cancer Res Treat. 2015 Aug;153(1):219-34. doi: 10.1007/s10549-015-3525-x. Epub 2015 Aug 8. Breast Cancer Res Treat. 2015. PMID: 26253945 - Differential expression of ten-eleven translocation genes in endometrial cancers.
Ciesielski P, Jóźwiak P, Wójcik-Krowiranda K, Forma E, Cwonda Ł, Szczepaniec S, Bieńkiewicz A, Bryś M, Krześlak A. Ciesielski P, et al. Tumour Biol. 2017 Mar;39(3):1010428317695017. doi: 10.1177/1010428317695017. Tumour Biol. 2017. PMID: 28349832 - 5-hydroxymethylcytosine: a new insight into epigenetics in cancer.
Ye C, Li L. Ye C, et al. Cancer Biol Ther. 2014 Jan;15(1):10-5. doi: 10.4161/cbt.27144. Epub 2013 Nov 19. Cancer Biol Ther. 2014. PMID: 24253310 Free PMC article. Review.
Cited by
- TET family proteins and 5-hydroxymethylcytosine in esophageal squamous cell carcinoma.
Murata A, Baba Y, Ishimoto T, Miyake K, Kosumi K, Harada K, Kurashige J, Iwagami S, Sakamoto Y, Miyamoto Y, Yoshida N, Yamamoto M, Oda S, Watanabe M, Nakao M, Baba H. Murata A, et al. Oncotarget. 2015 Sep 15;6(27):23372-82. doi: 10.18632/oncotarget.4281. Oncotarget. 2015. PMID: 26093090 Free PMC article. - Expression of the methylcytosine dioxygenase ten-eleven translocation-2 and connexin 43 in inflammatory bowel disease and colorectal cancer.
El-Harakeh M, Saliba J, Sharaf Aldeen K, Haidar M, El Hajjar L, Awad MK, Hashash JG, Shirinian M, El-Sabban M. El-Harakeh M, et al. World J Gastroenterol. 2022 Oct 28;28(40):5845-5864. doi: 10.3748/wjg.v28.i40.5845. World J Gastroenterol. 2022. PMID: 36353202 Free PMC article. - p53-Suppressed Oncogene TET1 Prevents Cellular Aging in Lung Cancer.
Filipczak PT, Leng S, Tellez CS, Do KC, Grimes MJ, Thomas CL, Walton-Filipczak SR, Picchi MA, Belinsky SA. Filipczak PT, et al. Cancer Res. 2019 Apr 15;79(8):1758-1768. doi: 10.1158/0008-5472.CAN-18-1234. Epub 2019 Jan 8. Cancer Res. 2019. PMID: 30622117 Free PMC article. - Deoxyribonucleic Acid 5-Hydroxymethylation in Cell-Free Deoxyribonucleic Acid, a Novel Cancer Biomarker in the Era of Precision Medicine.
Xu L, Zhou Y, Chen L, Bissessur AS, Chen J, Mao M, Ju S, Chen L, Chen C, Li Z, Zhang X, Chen F, Cao F, Wang L, Wang Q. Xu L, et al. Front Cell Dev Biol. 2021 Dec 10;9:744990. doi: 10.3389/fcell.2021.744990. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34957093 Free PMC article. Review. - Hydroxymethylation as a Novel Environmental Biosensor.
Dao T, Cheng RY, Revelo MP, Mitzner W, Tang W. Dao T, et al. Curr Environ Health Rep. 2014 Mar 1;1(1):1-10. doi: 10.1007/s40572-013-0005-5. Curr Environ Health Rep. 2014. PMID: 24860723 Free PMC article.
References
- Esteller M (2008) Epigenetics in cancer. N Engl J Med 358: 1148–1159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by funds from National Natural Sciences Foundation of China (No. 81090423, 81020108026, 81000966 and 81101630) and National Basic Research Program of China (973 Program, No.2010CB529406). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical