Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection - PubMed (original) (raw)
Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection
Michael B Francis et al. PLoS Pathog. 2013 May.
Abstract
Clostridium difficile spores must germinate in vivo to become actively growing bacteria in order to produce the toxins that are necessary for disease. C. difficile spores germinate in vitro in response to certain bile acids and glycine. In other sporulating bacteria, proteins embedded within the inner membrane of the spore sense the presence of germinants and trigger the release of Ca⁺⁺-dipicolinic acid (Ca⁺⁺-DPA) from the spore core and subsequent hydrolysis of the spore cortex, a specialized peptidoglycan. Based upon homology searches of known germinant receptors from other spore-forming bacteria, C. difficile likely uses unique mechanisms to recognize germinants. Here, we identify the germination-specific protease, CspC, as the C. difficile bile acid germinant receptor and show that bile acid-mediated germination is important for establishing C. difficile disease in the hamster model of infection. These results highlight the importance of bile acids in triggering in vivo germination and provide the first description of a C. difficile spore germinant receptor. Blocking the interaction of bile acids with the C. difficile spore may represent an attractive target for novel therapeutics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. Isolation of C. difficile germination-null mutants.
(A) Strategy to identify C. difficile ger phenotypes. Spores were generated (1) and purified (2). After purification, spores were germinated in BHIS medium supplemented with TA (3) and germinated spores heat-killed at 65°C (4). Spores that survived (4) were artificially germinated (5) before plating on BHIS medium (6). (B) C. difficile UK1 spores or C. difficile ger1 spores were serially diluted and spotted on BHIS medium supplemented with 0.1% TA or germinated by thioglycollate/lysozyme and were serially diluted and spotted on BHIS medium.
Figure 2. C. difficile ger isolates fail to initiate germination.
Purified C. difficile UK1 spores (A) or C. difficile ger1 spores (B) were suspended in BHIS medium (•) or BHIS medium supplemented with 5 mM TA (▪) or 50 mM TA (▴) and the initiation of germination was followed at A600. (C) DPA release from spores suspended in germination buffer supplemented with TA and glycine was analyzed at A270.
Figure 3. C. difficile cspC is essential for bile acid-mediated spore germination.
Purified C. difficile UK1 spores (A) or C. difficile JSC10 (cspC::ermB) spores (B) or C. difficile JSC10 (cspC::ermB) pJS123 (p_cspBAC_) (C) were suspended in BHIS medium (•) or BHIS medium supplemented with 5 mM TA (▪) or 50 mM TA (▴) and the initiation of germination was followed at A600. (D) DPA release from spores suspended in germination buffer supplemented with TA and glycine was analyzed at A270.
Figure 4. Mutations in C. difficile cspC can alter germination specificity.
Purified C. difficile JSC10 (cspC::ermB) pJS144 (p_cspBACG457R_) spores were suspended in BHIS medium (•) or BHIS medium supplemented with 1 mM chenodeoxycholic acid (▴) or 5 mM chenodeoxycholic acid (▾) or 10 mM TA (▪) and the initiation of germination was followed at A600.
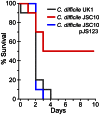
Figure 5. Bile acid-mediated germination is required for virulence.
Kaplan-Meier survival curve of clindamycin-treated Syrian hamsters inoculated with 1,000 spores of C. difficile UK1 or C. difficile JSC10 (cspC::ermB) or C. difficile JSC10 (cspC::ermB) pJS123 (p_cspBAC_). Animals showing signs of C. difficile infection (wet tail, poor fur coat, lethargy) were euthanized.
References
- McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, et al. (2005) An epidemic, toxin gene-variant strain of Clostridium difficile . N Engl J Med 353: 2433–2441. - PubMed
- Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, et al. (2010) The role of toxin A and toxin B in Clostridium difficile infection. Nature 467: 711–713. - PubMed
- Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, et al. (2009) Toxin B is essential for virulence of Clostridium difficile . Nature 458: 1176–9 doi:10.1038/nature07822. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases